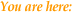 Home:
Meeting
Highlights: Posters
Home:
Meeting
Highlights: Posters

Evaluation
of Local and Systemic Disease Control Following Breast Conserving
Surgery After Neoadjuvant Anastrozole Treatment
Sahni S, Daltrey I, Renshaw L, Miller WR, Dixon JM
Anastrozole
is a one of the new generation of non-steroidal aromatase inhibitors.
In clinical trials it has been demonstrated that when used as second
line endocrine treatment for advanced breast cancer 1 mg of anastrozole
is more effective than megestrol acetate with a survival advantage
in favour of anastrozole. In the first line metastatic setting anastrozole
is associated with a survival advantage over tamoxifen in ER positive
patients. The Edinburgh Breast Unit has also studied anastrozole
used as neoadjuvant treatment in postmenopausal women with hormone
sensitive breast cancers.
To investigate
the outcome of patients treated by neoadjuvant anastrozole with
particular emphasis on local disease control in patients who initially
would have required mastectomy but who after primary anastrozole
treatment were suitable for breast conserving treatment.
- 26 postmenopausal
patients were entered into the study
- 13 were randomised
to receive a 1mg dose of anastrozole and 13 received a 10mg dose
Patient
Details are summarised in Table 1
- Patients
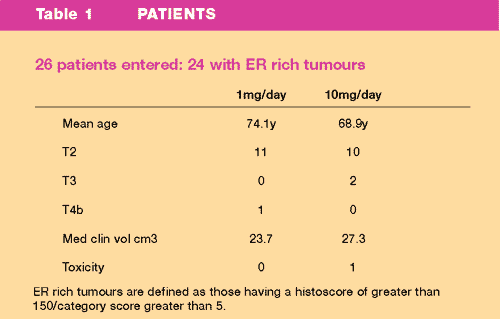
were treated with anastrozole for 3 months.
- Tumour size
and volume was monitored by monthly clinical and ultrasound measurements
and by mammography performed before and after the 3 month treatment
period
A summary
of the Treatment protocol is outlined in Figure 1
Patients were
enrolled in the study from March 1997 to June 1998.
- Response
was calculated using three-dimensional volume and actual changes
in tumour volume were calculated for clinical, ultrasound and
mammographic measurements.
| Results
of Neoadjuvant Anastrozole treatment
|
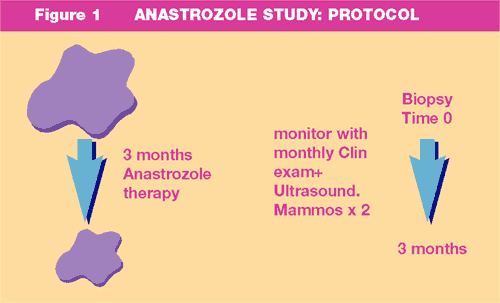
- Responses
as assessed by changes in tumour volume measured clinically, mammographically
and on ultrasound are shown in Table 2.
Click
on images below for larger image view in new window:
- Examples
of a mammographic response and changes on ultrasound during treatment
are shown in figures 2A, B, and C.
- An example
of a clinical response is shown in Figure 3. The ulcerated area
over the cancer are epithelialised during the three month treatment
period.
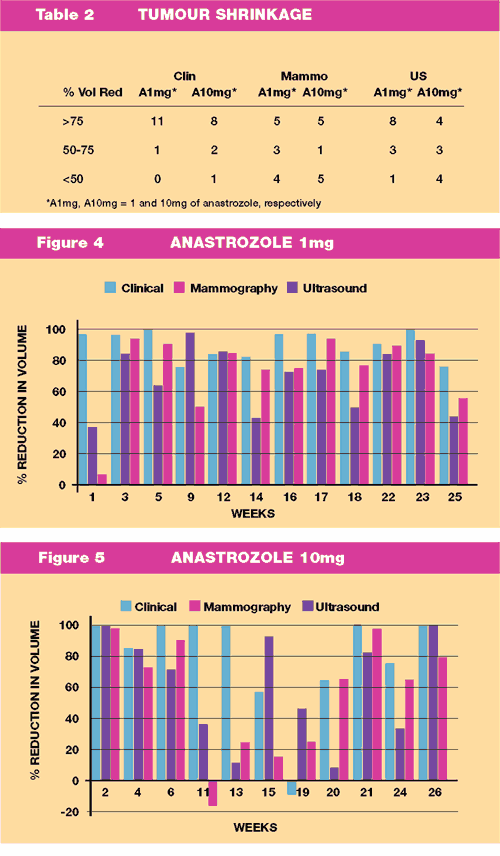
Summary of the Response data is presented in Table 2, Figure
4 and Figure 5.
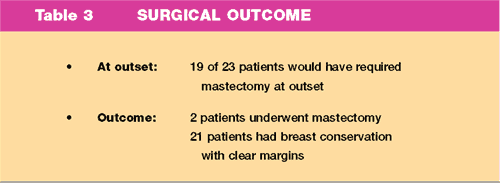
Surgical
Outcome
The numbers
of patients who would have required mastectomy at the outset of
treatment and the number of patients receiving treatment after 3
months anastrozole therapy is shown in Table 3. Axillary Surgery
was undertaken in all patients.
-
All patients
who had breast conserving surgery had complete tumour excision.
-
10 patients
were histologically node positive.
Treatment
after surgery
-
All patients
who had breast conserving surgery also had post operative radiotherapy.
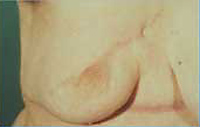
- The
patient with the locally advanced breast cancer shown in
Figure 6 is shown following completion of her local therapy.
- 3 patients
Ð all had 3 or more involved axillary nodes
- All
patients were continued on 1mg of anastrozole, the plan
being to continue this for 5 years
|
Click
on image below for larger image view in new
window:
|
| Figure
6 |
 |
Follow
up
All patients
have been carefully monitored by regular clinical examination and
yearly mammography.
Recurrences
-
There has
been 1 spot local recurrence 18 months post treatment treated
by reexcision followed by a change in endocrine therapy from
anastrozole to tamoxifen.
-
There have
been no systemic recurrences at a mean follow up period of 31
months.
-
3 patients
complained of individual side effects which included night sweats
24 months after starting adjuvant anastrozole weight gain (1)
and diarrhoea (1) 18 months after starting adjuvant anastrozole
. None of these side effects were severe but in an effort to
alleviate these symptoms all 3 patients were switched to tamoxifen
with some amelioration of symptoms.
- Neoadjuvant
anastrozole produces dramatic reductions in tumour volume over
a 3 month period in postmenopausal patients with ER rich breast
cancers. n Neoadjuvant anastrozole increases significantly the
number of patients with large operable or locally advanced breast
cancers who can be treated by breast con serving surgery.
- Local and
systemic control of disease in this elderly group of patients
with large or locally advanced breast cancers has been excellent
with only a single local recurrence.
- Adjuvant
anastrozole appears well tolerated and is likely to have contributed
to the excellent disease free and overall survival of this group
of patients.
- The use of
neoadjuvant endocrine therapy followed by breast conserving treatment
appears a reasonable option for postmenopausal patients with ER
rich large operable or locally advanced breast cancer.
- Further evaluation
of the role of anastrozole as neoadjuvant therapy in postmenopausal
women is indicated.
Top of Page |



