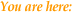 Home:
Meeting
Highlights: Posters
Home:
Meeting
Highlights: Posters

Rationale
for Proposed National Surgical Adjuvant Breast and Bowel Project
(NSABP) DCIS Trial
Tamoxifen versus Arimidex® (anastrozole) in Postmenopausal
Patients with Ductal Carcinome in Situ
Richard Margolese, MD
| Background:
Prior related NSABP trials |
NSABP B-17
Results
- Initial results
were reported in 1993 after a median duration of 43 months follow-up
and remain essentially unchanged through eight years of follow-up.

- All patients
benefited from radiation regardless of clinical or mammographic
tumor characteristics
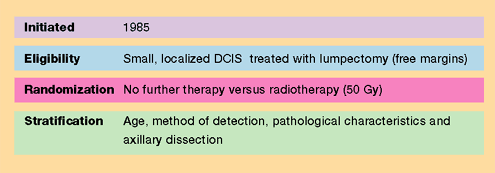
NSABP B-24
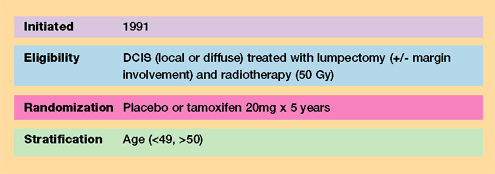
Results
- Women in
the tamoxifen group had fewer breast cancer events at five years
than did those on placebo (8.2% vs 13.4%, p=0.0009) —a relative
reduction of 37%.
- Tamoxifen
reduced invasive breast cancer by 43% (p=0.004).
- Ipsilateral
invasive breast cancer was reduced in the tamoxifen group by 44%
(p=0.03). This lowered the absolute rate from 4.2% to 2.1%. This
benefit was observed in premenopausal and postmenopausal women,
those with positive and negative tumor margins, and in patients
with and without comedonecrosis.
- Contralateral
breast cancer risk for invasive and noninvasive new cancers was
reduced by 52% (p=0.01).
Proposed
New NSABP DCIS Trial

Rationale
for the Trial
1. Giving a
longer duration of tamoxifen does not add additional benefit as
demonstrated by NSABP B-14.
2. Other SERMs
do not present an obvious therapeutic advantage to tamoxifen in
adjuvant treatment.
Justification
for Comparing Arimidex versus Tamoxifen
1. Mechanism
of action: In postmenopausal women, the major source of circulating
estrogens is peripheral conversion of androgens via aromatase enzyme
in fat, muscle and liver. Inhibition of aromatase thus reduces circulating
and possibly intratumoral estrogen levels.
2. Tamoxifen
has partial agonist effects. In the endometrium, this results in
increased incidence of endometrial cancer. Nonsteroidal aromatase
inhibitors (anastrozole and letrozole) have no intrinsic hormonal
activity. Endometrium effects are unknown. In bone, tamoxifen has
an agonist effect, which is beneficial in postmenopausal women.
Bone effects of aromatase inhibitors are unknown.
3. Third generation
aromatase inhibitors (nonsteroidal: anastrozole, letrozole. steroidal:
exemestane) are potent, highly specific agents with an improved
side effects/tolerability profile compared to the older agents such
as aminoglutethimide.
4. A large international
trial (ATAC) is comparing Arimidex to tamoxifen to a combination
of Arimidex and tamoxifen as adjuvant therapy of invasive breast
cancer. Initial results should be reported this year.
5. Direct comparisons
of third generation aromatase inhibitors to tamoxifen in advanced
breast cancer suggest at least equivalent antitumor effect and a
better toxicity profile, particularly related to thromboembolic
complications (see below).
ATAC Trial (Arimidex,
Tamoxifen and Combination)
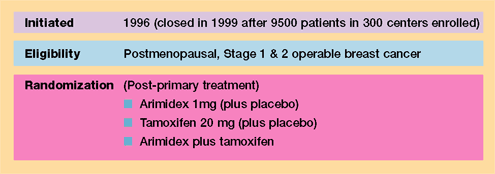
Randomized
trials comparing Arimidex to tamoxifen as first-line therapy for
metastatic disease (Trial 30—North American Trial and Trial
27 —European Trial)

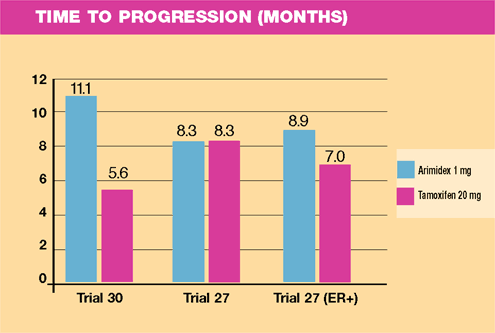
Key
Results of Trials 27, 30 (median follow-up of 18-months)
Efficacy
- Trial 30
demonstrated an advantage to Arimidex in time to progression and
clinical benefit rate. Trial 27 demonstrated equivalence. However,
the fraction of patients with known estrogen receptor-positive
tumors was greater in Trial 30 (89%) compared to Trial 27 (45%).
A trend to improved efficacy was noted in receptor- positive patients
in Trial 27.
- Conclusion:
Antitumor efficacy of Arimidex is at least equivalent to tamoxifen
in this setting.
Side Effects
and Toxicity Profiles (combined data from Trials 27 and 30)
- The side
effects were similar for both agents, with the exception of less
vaginal bleeding and fewer thromboembolic events in patients receiving
Arimidex. No differences were observed for weight gain, lethargy,
vaginal dryness, hot flash, GI disturbance, tumor flare or depression.

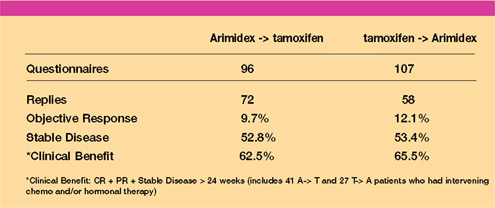
Crossover
Data
- The design
of Trials 27 (European) and 30 (North American) did not include
a planned crossover analysis to address whether the order of sequencing
endocrine agents differentially affects patient outcomes.
- Questionnaires
were sent to investigators, who provided data on patients who
had crossed over to a second endocrine treatment (either Arimidex
or tamoxifen) after failure on the first-line agent.
- Patients
who crossed over from Arimidex to tamoxifen or vice versa received
equivalent clinical benefit, regardless of whether or not they
had intervening chemotherapy, endocrine treatment or a chemohormonal
combination.
Response
on Crossover to Second Endocrine Therapy
- These findings
demonstrate that a significant fraction of patients will benefit
from tamoxifen after progressing on Arimidex and vice versa.
References
Fisher B, Dignam
J, Wolmark N et al. Lumpectomy and Radiation Therapy for the Treatment
of Intraductal Breast Cancer: Findings from National Surgical Adjuvant
Breast and Bowel Project B-17. Journal of Clinical Oncology 1998;16(2):441-452.
Fisher ER, Dignam
J, Tan-Chiu E et al. Pathologic findings from the National Surgical
Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17:
intraductal carcinoma. Cancer 1999;86(3):429-38. Fisher B, Dignam
J, Wolmark N et al. Tamoxifen in treatment of intraductal breast
cancer: National Surgical Adjuvant Breast and Bowel Project B-24
randomised controlled trial. Lancet 1999;353(9169):1993-2000.
Nabholtz JM,
Buzdar M, Pollak W et al. Anastrazole is superior to tamoxifen as
first-line therapy for advanced breast cancer in postmenopausal
women: Results of a North American multicenter randomized trial.
Journal of Clinical Oncology 2000;18(22):3758-3767.
Bonneterre J,
Thurlimann B, Robertson JFR et al. Anastrazole versus tamoxifen
as first-line therapy for advanced breast cancer in 668 postmenopausal
women: Results of the tamoxifen or Arimidex randomized group efficacy
and tolerability study. Journal of Clinical Oncology 2000;18(22):3748-3757.
Robertson J,
Buzdar A, Nabholtz J et al. Anastrozole (Arimidex®) versus tamoxifen
as first-line therapy for advanced breast cancer (ABC) in post-menopausal
(PM) women — prospective combined analysis from two international
trials. European Journal of Cancer 2000;36(S5):Abstract 219.
* Jewish
General Hospital, McGill University, Montreal Canada
Top
of Page
|



