|
 Home:
Meeting
Highlights: Posters
Home:
Meeting
Highlights: Posters

Recent
Changes in Physicians' Interpretation of Estrogen Receptor (ER)
Assay Results: Adjuvant Endocrine Therapy in Patients with low,
but Detectable ER
Jennifer Motley, MD
In
the last few years, a number of factors have caused physicians to
re-evaluate the use of adjuvant hormonal therapy based on estrogen
and progesterone receptor assay results.
- A report
by Harvey et al in May 1999 (1) evaluated the benefits of adjuvant
endocrine therapy (mainly tamoxifen) based on immunohistochemical
(IHC) assays for ER and PR. This retrospective study —based
on the San Antonio Breast Cancer tissue bank —compared IHC
to traditional ligand binding results in a series of 1982 patients
with close clinical follow-up. IHC proved to be a better predictor
of response to endocrine therapy, and patients with as few as
1% cells staining weakly for ER on IHC derived benefit from endocrine
therapy.
- A number
of prominent clinical trials, including two key studies from the
NSABP (2,3), have reported benefits of tamoxifen in high-risk
women and for patients with ductal carcinoma in situ, and many
such patients have being treated. This has increased awareness
of the chemopreventive effects of tamoxifen on contralateral breast
cancer in patients with invasive disease, who are at a risk of
similar magnitude to patients in the NSABP prevention trial (4).
The reduction in rate of second breast cancers in women receiving
adjuvant tamoxifen has not yet been clearly correlated with the
estrogen receptor assay result of the first cancer.
- The final
statement (5) of the November 2000 NIH Consensus Conference on
Adjuvant Therapy of Breast Cancer notes:
The
decision whether to recommend adjuvant hormonal therapy should
be based on the presence of hormone receptors, as assessed by
immunohistochemical staining of breast cancer tissue. If the
available tissue is insufficient to determine hormone receptor
status, it should be considered as being positive, particularly
in postmenopausal women. The small subset of women whose tumors
lack estrogen receptor protein but contain progesterone receptor
also appear to benefit from hormonal therapy...Adjuvant hormonal
therapy should be recommended to women whose breast tumors contain
hormone receptor protein, regardless of age, menopausal status,
involvement of axillary lymph nodes or tumor size. While the
likelihood of benefit correlates with the amount of hormone
receptor protein in tumor cells, patients with any extent of
hormone receptor in their tumor cells may still benefit from
hormonal therapy. Such treatment has led to substantial reductions
in the likelihood of tumor recurrence, second primary breast
cancer and death persisting for at least 15 years of follow-up.
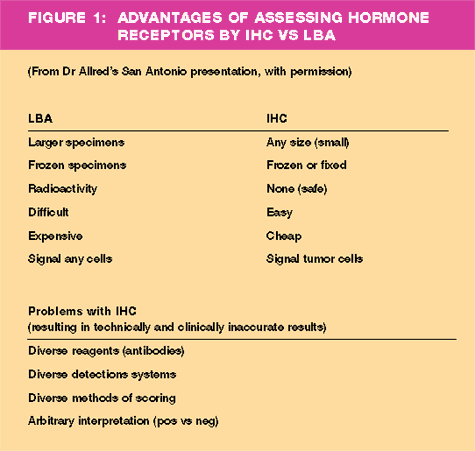
- At a presentation
during the December 2000 San Antonio Breast Cancer Symposium,
pathologist Craig Allred, MD, verbalized his concerns on quality
control in receptor assay technology:
"Estrogen
receptor and progesterone receptor assays are simply the most
important biomarkers in breast cancer today. Their assessment
is essential in deciding how to treat all patients with breast
cancer. However, the overall quality of the data that we're
getting from immunohistochemistry (IHC) today is, in fact, not
as good as what we got from the ligand binding assay (LBA).
That doesn't mean that it can't be, but right now I believe
that it's not... It's impossible to be very precise about what
the magnitude of this inaccuracy is, but I believe that we are
misdiagnosing at least five percent of patients that we see.
If you extrapolate that number to the 200,000 newly diagnosed
patients in the United States alone, that's 10,000 patients.
That's a lot, and in fact, I believe it's more on the order
of 10 or 15 percent."
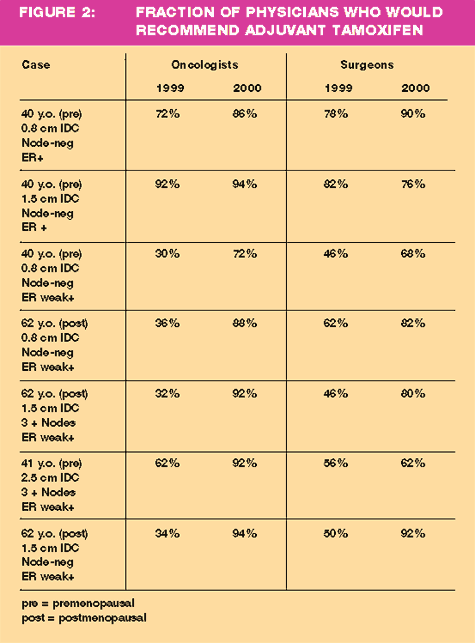
To
assess how this changing environment has affected clinical practice,
a random series of telephone surveys was conducted in June 1999
of 100 oncologists and 100 surgeons in the United States. A second
random survey was conducted in June 2000 of a different random sample
of 100 oncologists and 100 surgeons. Theoretical breast cancer case
scenarios were presented, and the physicians were asked how they
would manage such patients. The results show a marked change in
prescribing attitude during this time period with a significantly
greater fraction of physicians indicating they would recommend adjuvant
therapy with tamoxifen in women with both receptor-positive cancers
and tumors with low but detectable estrogen receptors. (Figure 2)
While physicians seem to be more proactive about utilizing endocrine
therapy in women with tumors with low, but detectable, estrogen
receptor assays, a number of recent reports from the pathology literature
suggests problems in performing and reporting these assays including
the following:
Rhodes A, Bharat
J, Balaton A, Barnes D, Anderson E, Bobrow L, Miller K Study
of Interlaboratory Reliability and Reproducibility of Estrogen and
Progesterone Receptor Assays in Europe. Am J Clin Path Jan 2001;
15(1). Full text version of this article: http://www.ajcp.com/rhodes0101ar.html#12
- This study
analyzed the specificity, sensitivity and reproducibility of IHC
assay for estrogen receptors (ER) and progesterone receptors (PR)
from 105 laboratories (66 of which had full participation) over
a two-year period.
- Receptor-positive
status was defined as 10% or greater of invasive tumor nuclei
staining by IHC.
- Only 36%
of the 66 labs that participated in all eight of the assessment
runs obtained reliable IHC assay results.
- Inadequate
microwave antigen retrieval time was found to be the major contributing
factor in the unreliable assays. While a majority of the labs
that were producing consistently reliable results were using pressure
cookers instead of microwaves, it was found that when microwave-heating
times were increased, IHC reliability scores improved significantly.
Rhodes A, Jasani
B, Barnes DM, Bobrow LG, Miller KD Reliability of Immunohistochemical
Demonstration of Oestrogen Receptors in Routine Practice: Interlaboratory
Variance in the Sensitivity of Detection and Evaluation of Scoring
Systems. J Clin Pathol 2000; 53: 125-130.
- This study
was designed to determine the rate of false negatives in IHC detection
of estrogen receptors.
- Histological
sections of breast cancers with low, medium and high estrogen
receptor expression were sent to 200 labs for evaluation.
- Only 37%
of laboratories detected the low estrogen-expressing tumors. About
80% of the labs were able to correctly detect the medium- and
high-expressing estrogen receptor cancers.
- Analyses
of the data showed that using a 1% or greater cut-off value for
determining receptor-positive status would significantly increase
the number of labs detecting all levels of ER expression (high,
medium and low).
- This study
was not able to identify specific variables predominantly responsible
for the differences in IHC sensitivity between the labs.
The two studies
discussed above point out the wide range of interlaboratory variability
in results and suggest that external quality assessment of the variables
involved in IHC assays could help standardize procedures and increase
reliability. Noting that close to one tenth of estrogen receptor-positive
patients have only 1-10% of estrogen receptor-positive staining
nuclei, and the second study proposes a 1% or greater staining threshold
for calling a cancer ER positive. A lower threshold may help to
"compensate for the slightly differing levels for IHC sensitivity
observed between laboratories."
While surgeons
and medical oncologists are being more proactive about utilizing
adjuvant tamoxifen in breast cancer patients with detectable but
low levels of estrogen receptor protein, pathology laboratories
may be providing misleading information that may result in undertreatment
of patients.
1.
Harvey et al. Estrogen receptor status by immunohistochemistry (IHC)
is superior to the ligand-binding assay (LBA) for predicting response
to adjuvant endocrine therapy in breast cancer. Journal of Clinical
Oncology 1999;17:1471-1481.
2.
Fisher B et al. Tamoxifen for prevention of breast cancer: report
of the National Surgical Adjuvant Breast and Bowel Project P-1 Study.
J Natl Cancer Inst 1998 Sep 16;90(18):1371-1388
3.
Fisher, B et al. Tamoxifen in treatment of intraductal breast cancer:
National Surgical Adjuvant Breast and Bowel Project B-24 randomised
controlled trial. Lancet 1999 Jun 12;353(9169):1993-2000
4.
Mamounas EP et al. Primary breast cancer as a risk factor for subsequent
contralateral breast cancer: NSABP experience from nine randomized
adjuvant trials. Breast Can Res Treat 1999, Abstract 15.
5.
Final NIH 2000 Consensus Statement on Adjuvant Therapy of Breast
Cancer: http://odp.od.nih.gov/consensus/cons/114/114_statement.htm
Top
of Page
|



