|
 Home:
Meeting
Highlights: Posters
Home:
Meeting
Highlights: Posters

The
UK Almanac Trial (MRC) - Early Results
Professor RE Mansel-Principal Investigator on behalf of the ALMANAC
Trialists Group
Sentinel Node
Biopsy (SNB) is rapidly becoming the surgical staging procedure
of choice to determine the status of the axillary nodes in the management
of breast cancer. Whilst there have been a large number of audit
studies confirming the validity of SNB in breast cancer, there are
no published randomized, controlled trials to validate this new
surgical technique. The ALMANAC trial (Axillary Lymphatic
Mapping Against Nodal Axillary Clearance)
is a two-phased, multi-centre, randomized trial being run in the
United Kingdom comparing SNB with standard axillary treatment in
the management of breast cancer.
The randomization
phase of the trial started in August, 2000 and as of January, 2000,
243 patients were recruited. The target for recruitment is 1,260
patients by the end of 2001.
We present our
early data of the audit phase of the above trial, which has been
funded by the Medical Research Council and has been completed in
9 centers.
In the audit
phase each of the 11 surgeons, whose results are presented here,
performed a SNB followed by a standard axillary procedure (clearance
or sampling) in 40 consecutive patients. All patients with breast
cancer in whom an axillary procedure was indicated were included
in the study. Following informed consent, the sentinel node was
localized using a protocol of a combination of a radiopharmaceutical
and blue dye (Patent blue V). The radiopharmaceutical used was Nanocoll
(Nycomed Amersham), 40 MBq if injected the day before surgery or
20 MBq if injected on the day of surgery. This was injected around
the tumour and was followed by a static lymphoscintiscan performed
around 3 hours after the injection. 16 patients did not have a lymphoscintiscan
due to logistical problems. Patent blue V was injected pre-operatively
around the tumour and the sentinel node was localized using a combination
of the blue dye and a hand held gamma probe. The SNB was followed
by the standard axillary procedure and all nodes including the sentinel
node were assessed by standard haematoxylin and eosin staining from
paraffin blocks.
The objective
of the audit phase was to standardize the surgical technique of
localizing the sentinel node in breast cancer and to assess the
learning curve involved in the introduction of a new surgical technique.
To facilitate these objectives, all surgeons in the trial attended
a course on SNB and in addition were proctored in the procedure
by the Principal Investigator of the trial.
The data presented
is from 440 patients (436 female, 4 male), of these 365 had palpable
lesions and 150 (34%) were screen detected. The mean tumour size
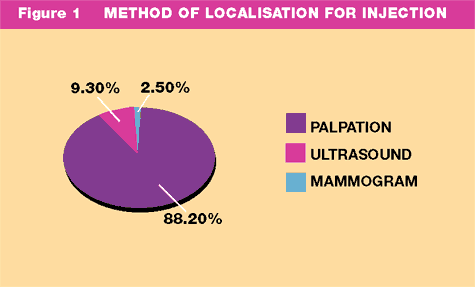
was 21 mms (range 1.7 - 100mms). Tumours were localized for injection
by palpation (88.2%), ultrasound (9.3%) and mammogram (2.5%) (Fig:1).
On the scintiscan, axillary drainage was seen in 68% of the cases
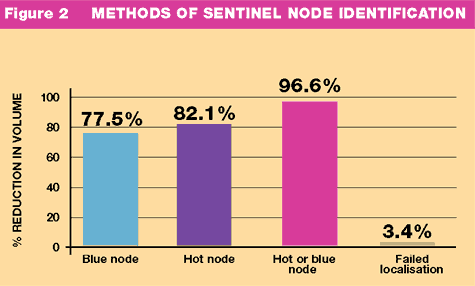
and internal mammary drainage in 8%. A sentinel node was successfully
identified in 425 patients (96.6%, Fig:2) and the mean number of
sentinel nodes removed was 2.2 per patient (range 1 - 8). The mean
time for completing a sentinel node biopsy was 17 minutes (range
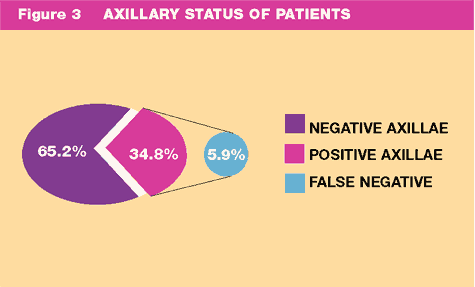
2 - 90 minutes). There were 153 (34.8%) patients with a positive
axillae, 9 of these patients had a false negative sentinel node,
giving a false negative rate of 5.9% (Fig:3).
This study shows
that after detailed instruction on the theory and a session of proctoring
by an experienced surgeon all the surgeons were able to achieve
a high success rate of locating the sentinel node with a low false
negative rate. These data represent the whole learning phase for
all the surgeons except the PI and one other who had performed some
80 procedures prior to undertaking the 40 case audit series. We
conclude that with adequate training sentinel node biopsy can be
performed to a high standard.
 |
ALMANAC
centres: Bangor, N.Wales; Cardiff*; Charing Cross, London*;
Derby; Edinburgh*; Guildford*; Huddersfield*; Hull; Leeds*;
Manchester*; Nottingham*; Portsmouth*; Rhyl, N.Wales; Swansea
*Centres that contributed data for this poster Trial Steering
Committee: Professor R Haward (Chairman), Professor D Cohen,
Professor P Ell, Dr I Ellis, Professor L Fallowfield, Professor
R Mansel, Dr R Newcombe, Dr E Rutgers, Professor C Woodman |
Top
of Page
|



