|
 Home:
Educational Supplement: Appendix
Home:
Educational Supplement: Appendix
Is
HER-2/neu a Predictor of Anthracycline Utility? No.
George
W. Sledge, Jr., M.D.
HER-2/neu is
important in the natural history of breast cancer. This importance
derives from HER-2/neu’s role in tumor growth, invasion, and
metastasis (the clinical summation of which is its role as a prognostic
factor in early-stage breast cancer) as well as its role as a predictor
of response to trastuzumab (Herceptin monoclonal antibody). These
roles for HER-2/ neu seem firmly established.
Three recent
American cooperative group trials have suggested that patients receiving
adriamycin are the most likely to benefit if their tumors overexpress
the HER-2/neu glycoprotein. This role of HER-2/neu, as a predictor
of anthracycline utility seems promising, and if confirmed clearly
would be of importance. But to what extent should we accept the
data presented to date?
Problems
in Positive Trials
Three trials
have reported positive results (Paik, Bryant, Park, et al., 1998;
Thor, Berry, Budman, et al., 1998; Ravdin, Green, Albain, et al.,
1998). Individually and collectively, however, these trials suffer
from potential and actual flaws. All data presented to date utilize
immunohistochemical techniques (IHC), and such techniques are well
known to suffer from methodological flaws. Even in good hands, the
correlation of these techniques with the “gold standard”
of fluorescence in situ hybridization (FISH) is only about 80 percent.
IHC techniques also have unknown correlations with each other, and
all three studies used differing antibodies and techniques. In each
case, tissue collection was retrospective. In one of the studies
(Thor and colleagues), the antibody utilized was switched mid-study;
in another (Paik and colleagues), the IHC technique was done on
old slides rather than off paraffin-embedded tissue.
Leaving aside
these potential methodological flaws, the analyses of the reported
trials leave something to be desired. All three involved retrospective
analyses and should therefore be viewed as hypothesis-generating
rather than as proof-of-principle studies. In the Cancer and Leukemia
Group B (CALGB) trial, an initial analysis reported positive results,
a second analysis with a somewhat larger number of patients failed
to reach statistical significance, and a third analysis combining
the two was once again positive. The data reported by SWOG involved
subset analyses involving a truly small number of patients, with
broad confidence intervals. Tests for interaction did not reach
statistical significance in any of the three trials.
Finally, what
are we to make of comparing the available trials? In the first trial
reported (CALGB), “high-dose” anthracycline-based therapy
was superior to “low-dose” anthracycline-based therapy.
The low-dose arm of the CALGB trial, however, utilized the same
dose intensity employed in the standard AC arm of NSABP B-11. Are
we to believe that the magnitude of difference between AC and CMF
in the NSABP trial is equal to the magnitude of difference between
that same dose and twice the dose of adriamycin? That seems unlikely.
Problems
With Consistency
Though most
doxorubicin-based adjuvant trials have reported positive results,
at least one epirubicin-based analysis reported no differential
benefit for HER-2 positivity (Untch, Konecny, Lebeau, et al., 1998).
That study compared a standard-dose epirubicin/cyclophosphamide
combination with a dose-intense epirubicin/cyclophosphamide regimen.
Only in the HER-2 negative subgroup was there a benefit for the
dose-intense regimen, a result that contrasts strikingly with the
CALGB results. Given that epirubicin is at least as beneficial in
the adjuvant setting as doxorubicin, how could one explain the absence
of an HER-2 related benefit?
Similarly, Colozza,
Gori, Mosconi, et al. (1999) evaluated the effect of HER-2 status
in a trial comparing epirubicin with CMF adjuvant chemotherapy in
patients with Stage I or II breast cancer. With a median followup
of 5.6 years and 266 patients available for HER-2 status, epirubicin
treatment had no significant impact on the outcome of patients with
HER-2 positive tumors.
One would expect
a consistency of effect across all stages of breast cancer if the
HER-2 relationship were real. We would not expect a predictive factor
to have a benefit in Stage II breast cancer but not in Stage III
or IV breast cancer, yet this is what we are asked to accept for
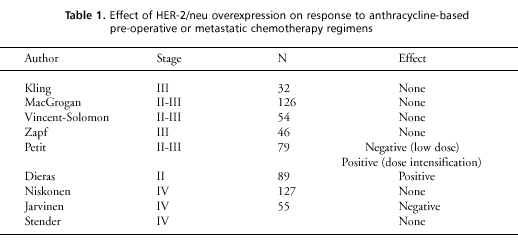
the HER-2/doxorubicin interaction. Numerous trials have examined
the effect of HER-2 overexpression on response to preoperative or
metastatic anthracycline-based regimens. These trials (summarized
in table 1) are inconsistent in their results, and generally fail
to demonstrate a positive relation. Although the number of patients
entered in these trials was small relative to the numbers of those
in adjuvant trials, the number of events is large, since virtually
every patient entered was measurable for response.
Biologic
Problems
If HER-2 overexpression
conferred sensitivity to doxorubicin in the clinic, one would expect
that a similar effect might be seen in the laboratory. Pegram and
colleagues tested this hypothesis by transfecting four breast cancer
cell lines with HER-2 and then exposing them to doxorubicin in vitro.
No alteration in chemosensitivity was observed in any of the transfected
breast cancer cell lines versus the parent cell lines, nor in a
related in vivo nude mouse xenograft model (Pegram, Finn, Arzoo,
et al., 1997).
Problems
With Extrapolation
The problem
with accepting the results seen in the available adjuvant trials
mirrors the problem with HER-2 testing in general. Immunohistochemical
analysis of HER-2 is only imperfectly correlated with fluorescence
in situ hybridization (FISH), and is subject to interobserver reproducibility
problems, problems with technique, and problems with tissue preservation.
None of the current trials used the same immunohistochemical techniques.
How then can we extrapolate the results to clinically available
testing kits?
Similarly, proponents
of the HER-2/doxorubicin link need to follow through on the logical
conclusions of this linkage. Two-thirds to three-quarters of breast
cancer patients are HER-2 negative. The same studies suggesting
a benefit for doxorubicin in HER-2 positive patients show a lack
of added benefit in HER-2 negative patients. Given the overview
demonstration of benefit for anthracycline-based regimens in the
adjuvant setting, are we really confident that doxorubicin can be
omitted in HER-2 negative patients?
Conclusion
Conclusion HER-2/neu
testing has many real benefits. These benefits should not blind
us to the real concerns surrounding the use of HER-2 as a therapeutic
predictor for anthracyclines. We currently lack a solid biologic
basis for the proposed linkage. The available positive studies have
real uncertainties. There are studies contradicting the linkage
in the adjuvant, neo-adjuvant, and metastatic settings. The cumulative
weight of these concerns calls the hypothesis into question. Until
more solid data emerges, HER-2 should not be accepted as a predictor.
References
Colozza M, Gori
S, Mosconi A, et al. erb-B2 expression as a predictor of outcome
in a randomized trial comparing adjuvant CMF vs single agent epirubicin
in Stage I-II breast cancer (BC) patients. [abstract]. Proc Am Soc
Clin Oncol 1999;18:70. Abstract.
Paik S, Bryant
J, Park C, Fisher B, Tan-Chiu E, Hyams D, et al. erbB-2 and response
to doxorubicin in patients with axillary lymph node-positive, hormone-receptor-negative
breast cancer. J Natl Cancer Inst 1998;90:1361-70. Abstract.
Pegram MD, Finn
RS, Arzoo K, Beryt M, Pietras RJ, Slamon DJ. The effect of HER-2/neu
overexpression on chemotherapeutic drug sensitivity in human breast
and ovarian cancers cells. Oncogene 1997;15:537-47. Abstract.
Ravdin P, Green
S, Albain K, et al. Initial report of the SWOG biological correlative
study of c-erbB-2 expression as a predictor of outcome in a trial
comparing adjuvant CAF T with tamoxifen (T) alone. [abstract]. Proc
Am Soc Clin Oncol 1998;17:97a. Abstract.
Thor AD, Berry
DA, Budman DR, Muss HB, Kute T, Henderson IC, et al. erbB-2, p53,
and efficacy of adjuvant therapy in lymph node-positive breast cancer.
J Natl Cancer Inst 1998;90:1346-60. Abstract.
Untch M, Konecny
G, Lebeau A, et al. Dose-intensification (DI) of anthracycline in
the adjuvant treatment of high risk breast cancer (HRBC) and c-erbB-2
overexpression. [abstract]. Proc Am Soc Clin Oncol 1998;17:103.
Abstract.
|

