|
 Home:
Educational Supplement: Appendix
Home:
Educational Supplement: Appendix
Overview
of the Six Randomized Adjuvant Trials of
High-Dose Chemotherapy in Breast Cancer
Karen
H. Antman, M.D.
There are six
reports of randomized trials of high-dose chemotherapy in high-risk
primary and metastatic breast cancer (see table 1). However, the
South African study has been discredited, and two of the remaining
studies randomized fewer than 100 patients and thus could not exclude
a survival difference of 30 percent. The Scandinavian study did
not compare high-dose versus conventional dose therapy. Thus, there
have been two reasonably large trials, but both with only about
3 years of median followup at the time of the last analysis.
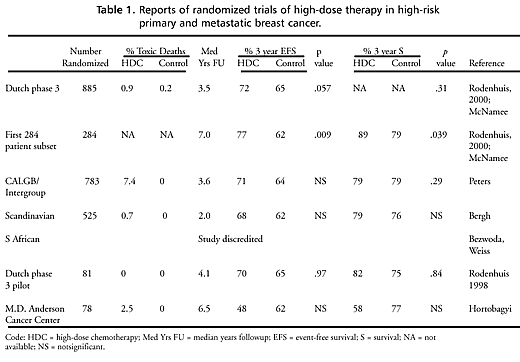
Mortality was
consistently low, in the 0 to 2.5 percent range, for the high-dose
regimens except for the CALGB/Intergroup study, which had a 7.4
percent toxic mortality rate. Mortality for the more conventional
dose arms was in the range of zero to 1 percent.
The Dutch trial
was the largest of the six studies (885 patients randomized) and
therefore had the greatest statistical power to detect modest differences
(Rodenhuis, Bontenbal, Beex, et al., 2000; McNamee, 2000). It compared
four courses of FEC (5-fluorouracil, epirubicin, cyclophosphamide)
to either an additional cycle of FEC or to CTCb (cyclophosphamide,
thiotepa, carboplatin) with stem cell support followed by surgery,
radiation, and tamoxifen for 2 years. In a planned analysis of the
first 284 patients, at a median followup of 6 years, disease-free
and overall survival were significantly better for the group receiving
high-dose therapy. In the study as a whole, the mortality was 1
of 443 patients on standard dose FEC and 4 of 442 on high dose CTCb.
At a median of 3 years followup, a trend (p=.057) emerged
favoring high-dose therapy.
The CALGB/Intergroup
study compared high versus intermediate dose cyclophosphamide, BCNU,
and cisplatin (CBP) after a CAF (cyclophosphamide, Adriamycin, 5
fluorouracil) induction (Peters, Rosner, Vredenburgh, et al., 1999).
Although the study can be criticized on grounds that intermediate
dose CBP is not a standard regimen, this design in scientific terms
is a pure comparison between high and intermediate dose CBP.
This first generation
BCNU-containing regimen (as noted earlier) had a 7.4 percent mortality
rate, which varied with the experience of the transplant center
and increased with patient age. Substantial pulmonary and hepatic
toxicity also occurred. With a median of 3.6 years of followup at
the time of presentation, the differences in the PFS and OS rates
in the two groups were not significant. Fewer events occurred than
would have been predicted from historical series, suggesting either
patient selection or an effect of the intermediate dose CBP. Significantly,
fewer relapses occurred in the high-dose arm, but the survival rate
of the two arms was similar at 70 percent because of the early toxic
mortality of 7.4 percent. Increased mortality neutralized this early
benefit. The study group was selected to have a tumor mortality
of ~80 percent. Survival in both arms will fall with time, and significant
differences may or may not emerge.
The Scandinavian
trial compared induction FEC followed by one high-dose cycle of
CTCb versus six additional cycles of moderately high dose FEC. The
doses (in mg/M2) of FEC were tailored to individual tolerance
up to 600 of 5-FU, 120 of epirubicin, and 1,800 of cyclophosphamide
per cycle. The planned cumulative doses for tailored therapy actually
exceeded that for the BMT arm. Therefore, this study assessed the
role of high-dose therapy as compared to intermediate-dose chemotherapy
with a higher cumulative dose (Bergh, 1999). Three percent of the
patients on the tailored dose arm developed leukemia or myelodysplasia,
compared with none on the marrow transplant arm.
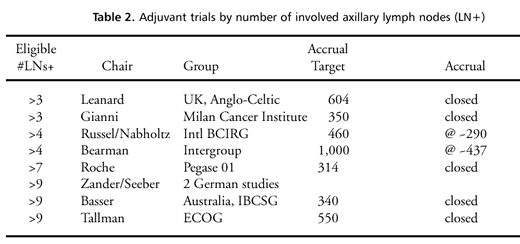
Ongoing or unpublished
randomized studies of high-dose therapy for breast cancer are shown
in table 2.
Additional followup
of the two large randomized trials and the completion of ongoing
randomized trials will provide more reliable data to determine the
role of high-dose chemotherapy regimens in the management of high-risk
primary breast cancer.
References
Bergh J. Results
from a randomized adjuvant breast cancer study with high dose chemotherapy
with CTCb supported by autologous bone marrow stem cells versus
dose escalated and tailored FEC therapy. Proc Am Soc Clin Oncol
1999;18:2a. Abstract.
Bezwoda WR.
Randomised, controlled trial of high dose chemotherapy versus standard
dose chemotherapy for high risk, surgically treated, primary breast
cancer. Proc Am Soc Clin Oncol 1999;18:2a. Abstract.
Hortobagyi GN,
Buzdar AU, Theriault RL, Valero V, Frye D, Booser DJ, et al. Randomized
trial of high-dose chemotherapy and blood cell autografts for high-risk
primary breast carcinoma. J Natl Cancer Inst 2000;92:225-33.
Abstract.
McNamee D. High
dose chemotherapy positive in breast cancer trial. Lancet 2000;355:1973.
Abstract.
Peters WP, Rosner
G, Vredenburgh J, et al. A prospective, randomized comparison of
two doses of combination alkyating agents as consolidation after
CAF in high-risk primary breast cancer involving ten or more axillary
lymph nodes: preliminary results of CALGB 9082/SWOG 9114/NCIC MA-13.
Proc Am Soc Clin Oncol 1999;18:1a. Abstract.
Rodenhuis S,
Bontenbal M, Beex L, et al. Randomized phase III study of high-dose
chemotherapy with cyclophosphamide, thiotepa and carboplatin in
operable breast cancer with 4 or more axillary lymph nodes. [abstract].
Proc Am Soc Clin Oncol 2000;19:74. Abstract.
Rodenhuis S,
Richel DJ, van der Wall E, Schornagel JH, Baars JW, Konniq CC, et
al. Randomised trial of high-dose chemotherapy and haematopoietic
progenitor-cell support in operable breast cancer with extensive
axillary lymph node involvement. Lancet 1998;352:515-21. Abstract.
Weiss RB, Rifkin
RM, Stewart FM, Theriault RL, Williams LA, Herman AA, et al. High-dose
chemotherapy for high-risk primary breast cancer: an on-site review
of the Bezwoda study. Lancet 2000;355:999-1003. Abstract.
|
|

