| You
are here: Home: BCU 6|2001: Section 3
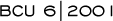
Section 3
New Trends in Adjuvant Systemic Therapy
CA VS FAC
This discussion goes back a long way in history. Gabe Hortobagyi
and the MD Anderson group developed FAC 63 - 66 at the
same time Sid Salmon and I developed A C 67,68. The NSABP
rediscovered A C and “modernized” it, although the dose
of cyclophosphamide we originally used was a little bit higher than
the current dose.
Another big issue is the correct number of treatments of A C 69
. Is six cycles better than four? In the randomized trials, four
cycles of AC is as good as anything else available in the adjuvant
setting. MD Anderson almost never gives less than six or eight treatments,
and they feel uncomfortable recommending four treatments, while
the rest of us — including the NSABP — are very comfortable
using four treatments in the right setting for the right group of
patients. Some argue that maybe that’s not enough treatment,
but that needs to be settled by a clinical trial of six or eight
cycles versus four. The old Sydney Farber trial of five versus 10
courses of AC was grossly underpowered to show any difference. ECOG
is planning a trial of six versus four courses of AC. The problem
with using a lot more doxorubicin is the markedly increased risk
of cardiac toxicity. With four courses, the risk is quite low.
—Stephen Jones, MD
Select Publications
ADJUVANT TAXANES
The issue of adjuvant taxanes is very controversial at the moment
70-79. Our group and others are in the midst of trials
where AC-paclitaxel is the standard treatment arm. In my own practice,
for node-positive, ER-PR negative patients, I give AC-paclitaxel.
On the other hand, if I had a patient with one or two positive nodes
who is ER-PR positive, I can’t in my own good conscience at
this point give them AC-paclitaxel, and I will give just four courses
of AC.
We have done some studies using docetaxel in the adjuvant setting,
and we presented some data at ASCO this year. We studied four cycles
of AC versus four cycles of docetaxel/cyclophosphamide — with
no doxorubicin. There was significantly less major toxicity with
the docetaxel/cyclophosphamide and, so far, equal efficacy. The
rest of the world is moving to anthracycline-taxane combinations,
and that may be where this goes. But we looked at an adjuvant regimen
that removed the anthracycline.
—Stephen Jones, MD
“The nonanthracycline
regimen of TC is a well-tolerated adjuvant treatment associated
short-term with fewer recurrences than standard AC. Longer follow-up
will be necessary to assess the overall efficacy and long-term toxicity
of the TC regimen compared to AC.”
—Jones SE et al. Proc ASCO 2001; Abstract
128.
One of the major issues in the adjuvant treatment of breast cancer
is the taxanes. We’re going to continue to receive follow-up
data on CALGB-9344 (AC followed by paclitaxel or control) and NSABP
B-28. Both of these trials have follow-up of less than five years,
and I think we’ll probably see an update within a year or two
to help us figure out whether taxanes add anything to further reduce
the risk of recurrence or death from breast cancer in the adjuvant
setting.
I’m basically of the opinion that ultimately the taxane data
will prove positive. Right now in a nonprotocol setting, I give
taxanes — usually paclitaxel — to node-positive patients.
There are certain exceptions to this rule. For example, in young
women concerned about fertility, I tend to use more CMF or AC, because
I think there’s a higher rate of amenorrhea with taxanes.
I’ve been very lucky in that I’ve been pretty much able
to put all of my node-positive patients on the Intergroup trial,
which does have a taxane. That makes it easy. Another big Intergroup
trial is A C versus doxorubicin-docetaxel. It will be interesting
to see if that adds anything in the node-negative setting. And there’s
another SWOG trial looking at every-two-week versus every-three-week
chemo, looking at the dose-dense chemotherapy concept.
If there is an advantage to shortening the interval, it may shift
how we treat patients. There are also several high-dose trials,
which will probably be reported in the next two to three years.
—Linda Vahdat, MD
AROMATASE INHIBITORS IN THE ADJUVANT SETTING
I think that we are moving very quickly to using AIs in the adjuvant
setting 80-89. The ATAC Trial has 9200 women enrolled,
and the first results will be presented at the upcoming San Antonio
meeting. There are also other trials under way, all large studies
with 4000+ patients. I’d be very surprised if the AIs don’t
show superiority to tamoxifen in these studies.
I see oncologists using an AI rather than tamoxifen in the adjuvant
setting for reasons such as patient intolerance or a fairly recent
DVT or something like that. I haven’t quite made that leap
of faith in my practice, but I’m a lot closer now than I was
a year ago. And certainly, if someone had a history of a DVT four
years ago, I might think about the use of an AI.
—Stephen Jones, MD
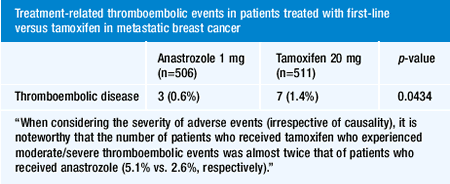
In terms of extrapolating from the ATAC trial outcome to other
aromatase inhibitors, I think we need to “dance with the data
that we see.” If the trial shows that anastrozole is superior
to tamoxifen and/or less toxic, then our obligation is to use anastrozole
until we have equally convincing data from clinical trials comparing
the other aromatase inhibitors to tamoxifen. I personally would
just stick with the one upon which the data is based and not extrapolate.
I have had several situations where I have substituted an aromatase
inhibitor for tamoxifen in the adjuvant setting. Two were patients
with thrombotic histories and known mutations. There are three cases
in which I’ve made the change over to an aromatase inhibitor
because patients could not tolerate tamoxifen.
—Kevin Fox, MD
It ’s important to maintain as close to standard therapy as
possible in the adjuvant setting, because you don’t want to
compromise the chance of cure. But, we certainly know that there
are some patients intolerant of tamoxifen or in whom tamoxifen is
contraindicated — those are the patients that I will give an
aromatase inhibitor to, even without long-term adjuvant data. Those
trials are in the process of being done, but certainly you can consider
extrapolating metastatic data to the adjuvant setting, at least
to say that you wouldn’t expect it to be any worse.
—Linda Vahdat, MD
TRIALS OF HIGH-DOSE CHEMOTHERAPY IN THE ADJUVANT SETTING
There was an excellent Intergroup adjuvant trial that closed in
February. It took patients with four or more positive lymph nodes
and randomized them to every-two-weeks doxorubicin, paclitaxel and
cyclophosphamide for three cycles at higher than standard doses,
versus AC with the doxorubicin followed by transplant. We put a
lot of patients on that study. Currently, for high-risk adjuvant
patients with ten or more positive nodes or stage III or inflammatory
cancer — we put them on our phase II institutional trial, which
is basically STAMP-5 followed by immunotherapy.
—Linda Vahdat, MD
FERTILITY PRESERVATION IN YOUNG WOMEN ON CHEMOTHERAPY
We are interested in fertility preservation in young women receiving
adjuvant systemic chemotherapy 97-102 . There is a simple
clinical paradigm that if you suppress ovarian function before chemotherapy,
then upon completion of chemotherapy, the patient stands a better
chance of retaining her menses and thus bearing children 103,104
. We have been using leuprolide to prevent amenorrhea in women receiving
adjuvant chemotherapy who have a particular interest in child-bearing.
We start the leuprolide a week prior to chemotherapy and give it
with each chemotherapeutic cycle. When chemotherapy ends, leuprolide
ends. We have found that menses return in an average of five to
six months. We’ve piloted the concept and have recorded data
in 17 women, and we’ve found that ovarian function can be preserved.
The obvious question of whether or not it would have been preserved
without the leuprolide is unanswerable and will require a randomized
trial.
—Kevin Fox, MD
“All patients became amenorrheic
by the second cycle of chemotherapy. Menstrual periods resumed in
all patients within 12 months of the completion of chemotherapy,
with a mean time to recovery of 4.9 months (range 2-12 months).
Twelve of 13 patients report regular cycles, and one reports irregular
menses at 16 months from completion of treatment. Four patients
also taking tamoxifen continue regular menses. ... Although the
probability of CRA (chemotherapy-related amenorrhea) in this cohort
of patients is low without leuprolide, the maintenance of regular
post-treatment menses in most of our patients is encouraging.”
—Fox K R. Proc ASCO 2001; Abstract
98.
“ It’s a shame that when you
look at the adjuvant therapy literature and you look for the incidence
of premature ovarian failure, it's only reported in one-third of
adjuvant therapy trials, and that is the single most important permanent
complication of adjuvant chemotherapy for premenopausal women. For
the newer drugs like paclitaxel or docetaxel we have no idea what
they do to ovarian function. We have a lot of data on the white
blood cell count, which always recovers, and so on and so forth,
but we don't know about this very very important toxicity. ”
— Melody Cobleigh, MD
Breast Cancer Update #4, 1998
Select Publications
|
