| You are here: Home: BCU 1|2002: Dr. Daniel R. Budman, MD
Edited Comments by Dr Budman
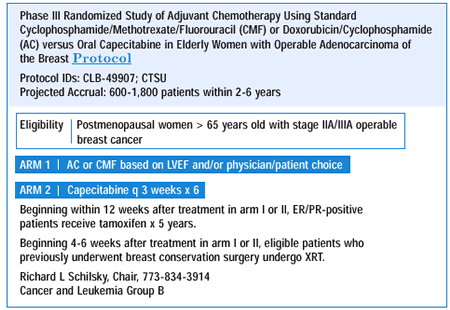
ADJUVANT TAXANES
In node-positive, estrogen receptor-negative patients — where CALGB 9344 still shows some benefit — we have been using four cycles of AC followed by four cycles of paclitaxel. Several members of our group have switched to six cycles of FEC, based upon the Canadian experience showing that it is better than CMF. We do not utilize taxanes in estrogen receptor-positive patients, since the trial did not show any benefit in that subset. There's no information currently available that will satisfy me that node-negative patients benefit from taxanes, and I would be very reluctant to offer it to them off protocol.
MECHANISMS OF ACTION OF TAMOXIFEN VERSUS AROMATASE INHIBITORS
There are important differences in the mechanism of action between tamoxifen and anastrozole. The aromatase inhibitors may allow you to abrogate the autocrine properties of breast cancer cells and offer another dimension of activity beyond antiestrogens. In the metastatic setting, the aromatase inhibitors are exceedingly welltolerated, with superb, very durable responses and good quality of life. Unless the AIs cause tremendous osteoporosis, I suspect they will replace antiestrogens.
CAPECITABINE: A RATIONALLY DERIVED AGENT
Capecitabine is a very intriguing, novel, chemically synthesized drug. The basis of its synthesis actually goes back to the 1980s, when various researchers described a technique called retrometabolic engineering. Capecitabine is a pro-drug, which undergoes enzymatic activation and can concentrate fluoropyrimidines in the tumor three to tenfold, to give potentially higher efficacy and selectivity due to this very clever engineering. The objective is to keep a very high concentration of the active drug — 5-FU — in the tumor and hopefully lessen host toxicity, thus increasing the therapeutic index.
Secondly, combined with other drugs such as taxanes, which upregulate thymidine phosphorylase — the enzymatic step to convert capecitabine into its active form — you can get curative potential. Using 5-fluorouracil under the same conditions, you do not.
AVOIDANCE OF HAND-FOOT SYNDROME THROUGH DOSE REDUCTION OF CAPECITABINE
Hand-foot syndrome is a dose-related side effect of capecitabine, perhaps in part due to the polymorphism of DPD, the enzyme that degrades 5-FU. Retrospective data from Joyce O'Shaughnessy demonstrated that two grams per meter squared per day was an acceptable dose, and I start very elderly patients out at 1,500 milligrams per meter squared per day. At these doses I've encountered very few instances of hand-foot syndrome in my practice. It's also important to educate patients about what to expect from a drug and what to look out for. I instruct patients to discontinue capecitabine if they experience redness in their hands or feet. I also emphasize that toxicity does not mean that they are going to have a response. In fact, some patients who have had the best responses have had no symptoms at all. I had one patient who travels extensively who has been on single-agent capecitabine for six months without any toxicity whatsoever — for her, it's been a godsend.
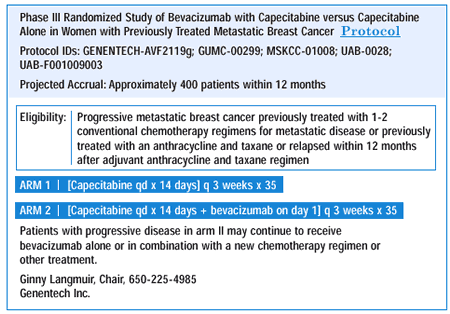
DOCETAXEL-CAPECITABINE STUDY: QUALITY OF LIFE
There is evolving evidence from a large randomized Phase III trial of over 500 patients that patients failing anthracycline therapy can maintain quality of life and increase disease-free and overall survival with the combination of docetaxel and capecitabine. In fact, the response and survival curves now show more benefit than they did when the data was initially presented. This is a standard of care that we have to look at carefully.
Quality of life is a critical issue in treating advanced disease. If we prolong duration of response without a reasonable quality of life, we are kidding ourselves. The docetaxel-capecitabine study was one of the few clinical trials where quality of life was a major endpoint. A rigorous quality-of-life measurement was used before and during the study, showing that patients receiving combination docetaxelcapecitabine actually had a better quality of life than patients receiving full-dose docetaxel alone. Although this may seem paradoxical, my interpretation is that if a patient has a response — and for example, her fungating tumor is gone — she can get out of bed, walk around and go to work, then obviously, there’s a benefit.
LACK OF CROSSOVER IN THE DOCETAXEL-CAPECITABINE STUDY
In the docetaxel-capecitabine study, only 17 percent of patients randomized to docetaxel crossed over to capecitabine after progression. However, a crossover in this trial would have been very difficult to accomplish. These were a tough group of patients. They had failed anthracyclines either in the adjuvant setting or in the metastatic setting, and, two-thirds of them had failed it in the metastatic setting. They had all received alkylating agents. Three-quarters had already received fluoropyrimidines — so they already had failed 5-FU to a great degree.
The take-home message from the study is that if you have a higher response rate, a better quality of life, and a longer duration of survival with the combination, you probably can't do as well with a sequential regimen, because you're going to have a higher tumor burden. You're going to have more morbidity. We know that if you can reduce tumor burden, then — as a group — you usually have better performance and better quality of life. A higher response rate with longer survival is obviously an advantage.
Select References
|
