|
You are here: Home: BCU 1|2002: Robert W Carlson, MD
FULVESTRANT IN PREMENOPAUSAL WOMEN
There is no reason to believe that fulvestrant would not be effective in premenopausal women. Although we do not yet have long-term disease-oriented clinical trials, the agent appears to have acceptable toxicity in short-term trials in premenopausal women. I would still, however, be cautious about considering this agent in premenopausal populations outside the confines of a clinical trial.
In preclinical systems, fulvestrant has limited effects on bone density and serum lipids. The data in humans are quite early, and those questions will have to be addressed by clinical trials. Certainly, these issues are especially important if fulvestrant is moved into the adjuvant setting, as we certainly expect it to be, because women in this setting are likely to be long-term survivors in whom bone and cardiovascular events are of substantial concern.
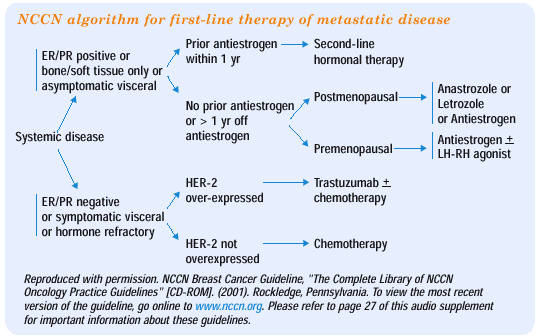
NCCN GUIDELINE ON FIRST-LINE HORMONAL THERAPY METASTATIC DISEASE
The nonsteroidal aromatase inhibitors are superior to tamoxifen as first-line treatment in metastatic disease. Therefore, in the current NCCN guideline, the use of aromatase inhibitors has been moved forward to a first-line option for postmenopausal women with hormoneresponsive breast cancer. Tamoxifen also remains a first-line option, because we have such a preponderance of data using tamoxifen in this setting that the panel did not want to remove it as a first-line therapy.
Only the nonsteroidal aromatase inhibitors — anastrozole and letrozole — are included in the first-line guideline. Although I have no criticism of practitioners who prefer letrozole, I tend to use anastrozole. It was the first of the selective aromatase inhibitors available, and it's one that I became very comfortable with. I personally use anastrozole almost exclusively in my practice.
The steroidal aromatase inhibitor, exemestane, is not included in the guideline as a first-line hormonal agent, because we don't have good comparative data yet available from large randomized studies.
In premenopausal women, the NCCN panel thought it was premature to consider combining an LH-RH agonist/antagonist with an aromatase inhibitor. I personally believe that this is a very important area for future research and would anticipate randomized trials of this combination. The superiority of the aromatase inhibitors in postmenopausal women does suggest that this may be a very effective strategy in premenopausal women, but at this point, the evidence is simply lacking to support that approach.
|
2002 NCCN PRACTICE GUIDELINE: HORMONAL THERAPY FOR METASTATIC DISEASE
Women considered to be appropriate candidates for initial hormonal therapy for treatment of recurrent or metastatic disease include those whose tumors are estrogen- and/or progesterone-positive, those with bone or soft tissue disease only or those with limited, asymptomatic visceral disease. In postmenopausal women with prior antiestrogen therapy and who are within one year of antiestrogen exposure, recent evidence supports the use of a selective, nonsteroidal aromatase inhibitor such as anastrozole or letrozole as the preferred second-line therapy (Buzdar et al, 2001; Buzdar et al, 1998). For postmenopausal women who are antiestrogen naïve or who have not been exposed to antiestrogen therapy for greater than one year, the selective, nonsteroidal aromatase inhibitors appear to have superior outcomes compared with tamoxifen, although the differences are modest (Bonneterre et al, 2000; Mouridsen et al, 2001; Nabholtz et al, 2000; Vergote et al, 2000). Therefore, either tamoxifen or an aromatase inhibitor is an appropriate option.
Reproduced with permission. NCCN Breast Cancer Guideline, "The Complete Library of NCCN Oncology Practice Guidelines" [CD-ROM]. (2001). Rockledge, Pennsylvania. To view the most recent version of the guideline, go online to www.nccn.org. Please refer to page 27 of this audio supplement for important information about these guidelines. |
ASSESSING HER2 STATUS
The NCCN guidelines call for HER2 testing of all breast cancers; however, this year we were much more specific than in the past. We call for HER2 testing by IHC, and if the IHC result is 2+ by the HercepTest™, we call for FISH analysis. This is primarily because in the metastatic setting, when you're looking for benefit or lack thereof from trastuzumab, FISH-positivity is by far the best predictor of responsiveness. Women whose breast cancers are IHC 3+ by the HercepTest™ are almost all FISH-positive, while those that are IHC 0 or 1+ are almost always FISH-negative. This is based upon the study reported by Chuck Vogel, which looked at trastuzumab as a single agent and found very good rates and long duration of response in those women who were either IHC 3+ or IHC 2+ and FISH-positive.
TRASTUZUMAB WITH OR WITHOUT CHEMOTHERAPY
In HER2-positive patients in the metastatic setting, where hormonal therapy is not selected as initial therapy, the guideline calls for the use of either single-agent trastuzumab or trastuzumab in combination with chemotherapy. We are fairly inclusive in terms of the selection of the chemotherapeutic agent. Although the highest level evidence available is for trastuzumab in combination with paclitaxel, the guideline acknowledges that there is nonrandomized, lower level evidence looking at trastuzumab in combination with docetaxel, vinorelbine, cisplatin and carboplatin. So, in the guideline, we do allow for the utilization of trastuzumab in combination with any of those agents.
|
2002 NCCN PRACTICE GUIDELINE: ALGORITHM FOR HER2 ASSESSMENT & SELECTION OF PATIENTS TO RECEIVE TRASTUZUMAB
Patients with tumors that overexpress HER2/neu may derive benefit from treatment with trastuzumab as a single agent or in combination with selected chemotherapeutic agents. The optimal method of selecting the subset of patients most likely to benefit from trastuzumab is rapidly evolving. When tested by the DAKO HercepTest, IHC staining of 2+ or 3+ appears to correlate with disease response to trastuzumab. However, benefit from trastuzumab treatment in patients with breast cancer IHC 2+ for HER2/neu appears to be limited to those tumors that are FISH-positive for HER2/neu amplification. Therefore, the panel recommends selecting patients for trastuzumab who have tumors either IHC 3+ for HER2/neu by the HercepTest or IHC 2+ for HER2/neu by the HercepTest and FISH-amplified (Field, 2001; Tubbs, 2001; Wang, 2000). Patients with tumors IHC 0 or 1+ for HER2/neu have very low rates of trastuzumab response, and therapy with trastuzumab is not warranted. In patients with metastatic or recurrent breast cancer whose tumors overexpress HER2/neu, trastuzumab as a single agent (Cobleigh, 1999; Vogel, 2001) or in combination with selected chemotherapeutics (Slamon, 2001) may be considered. A single randomized trial demonstrates benefit from adding trastuzumab to paclitaxel chemotherapy in patients with IHC 2+ or 3+ for HER2/neu. Early non-randomized data are available supporting the addition of agents such as docetaxel, vinorelbine and platinum compounds in combination with trastuzumab. The panel believes the 27% frequency of significant cardiac dysfunction in patients treated with the combination of trastuzumab and doxorubicin/ cyclophosphamide chemotherapy is too high for use of this combination outside the confines of a prospective clinical trial (Slamon, 2001).
Reproduced with permission. NCCN Breast Cancer Guideline, “The Complete Library of NCCN Oncology Practice Guidelines” [CD-ROM]. (2001). Rockledge, Pennsylvania. To view the most recent version of the guideline, go online to www.nccn.org. Please refer to page 27 of this audio supplement for important information about these guidelines. |
Page 2 of 3
Previous Page | Next Page
|
|
