| You are here: Home: BCU 1|2002: Edith A. Perez, MD
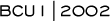
ADJUVANT CLINICAL TRIALS OF TRASTUZUMAB
Intergroup trial 9831 is an adjuvant study that was activated in May 2000. We have enrolled 700 patients with node-positive, HER2-positive breast cancer. This trial will join the three other worldwide studies being conducted to help answer the question of whether trastuzumab adds benefit to chemotherapy in this group of poor-prognosis women. We are also testing the question of whether trastuzumab should be used sequentially or concurrently with chemotherapy.
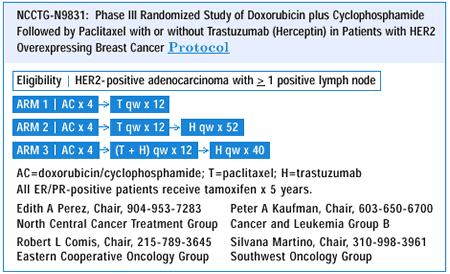
NSABP B-31 has very similar eligibility criteria. The NSABP uses paclitaxel every three weeks, while we are utilizing paclitaxel weekly. Another difference between the two trials is that the NSABP starts tamoxifen — if indicated — concurrent with AC, whereas in the
Intergroup trial, tamoxifen is started after the completion of the six months of chemotherapy. Additionally, we are submitting an amendment to our protocol to administer trastuzumab once every three weeks, whereas NSABP will maintain weekly trastuzumab for one year.
If someone uses adjuvant trastuzumab outside of a clinical trial setting, they’re essentially shooting in the dark. We do not yet understand for how long this therapy should be given, what schedule should be used in combination with chemotherapy, and the potential risks or benefits the patients may derive from such treatment.
CARDIOTOXITY AND TRASTUZUMAB
Both adjuvant trastuzumab trials — NCCTG N9831 and NSABP B-31 — are carefully attending to cardiac tolerability. At this time, no red flags have been raised. In our adjuvant trial, we have attempted to ameliorate the risk of cardiotoxicity by not using trastuzumab concurrently with anthracyclines and by limiting the dose of doxorubicin to 240 milligrams per meter squared. In the pivotal trastuzumab metastatic study, the increased risk of cardiotoxicity in terms of congestive heart failure was seen when the cumulative dose of doxorubicin was greater than 300 milligrams per meter squared.
We are accumulating data to help us understand what AC chemotherapy leads to in terms of ejection fractions, because this has never been investigated thoroughly. We are developing a companion cardiac tolerability study, and the NSABP will be conducting a similar study as well. We are attempting to determine whether we can find plasma factors that predict for clinical cardiotoxicity. There’s a lot of cardiology literature regarding the potential value of various factors, and we’re going to look at these in a prospective fashion to see if any correlate with clinical outcome.
We are also going to look at the correlation between ejection fractions obtained by MUGA versus echocardiogram, evaluating ejection fractions before study entry, after AC, after paclitaxel, and nine and 18 months into treatment. If we see more than 5% cardiotoxicity in the investigational arms compared to the standard arm, we will stop the trial.
DISCORDANCE IN THE ASSESSMENT OF HER2 STATUS
There is a significant problem with HER2 testing in the community. When we designed our adjuvant study — evaluating trastuzumab in combination with chemotherapy — we included a plan for central analysis of HER2 status. Unfortunately, when we looked at the initial 119 patients, we found a high level of discordance in HER2 testing by IHC, and even by FISH, when comparing measurements in the community versus central testing.
We found discordant results in six of the nine FISH test assays. These were FISH-positive in the community, but FISH-negative in the central lab. The number of patients is very, very small to date, so we cannot conclude that FISH is a bad test to be performed in the community, but we need to look into why this discordance occurs. IHC concordance between the community and central laboratories was about 75 percent.
We've done another study of HER2 testing, based on 1,500 specimens sent to Mayo medical laboratories over a five-month period. We took 213 specimens labeled as HER2 2+ and evaluated them for protein overexpression and gene amplification, and we found that only 12 percent of the tumors scored as 2+ by the HercepTest™ actually were FISH-positive.
CONTINUATION OF TRASTUZUMAB AFTER DISEASE PROGRESSION IN THE METASTATIC SETTING
My standard practice is to use trastuzumab until progression or toxicity. Whether it should be continued after disease progression is an issue we’re wrestling with on a day-to-day basis, and nobody knows the answer. We will join Dr Pusztai from MD Anderson in
his trial to help us answer this question in patients who have progressed on a taxane-trastuzumab combination. The randomization will be to continue on trastuzumab and add vinorelbine or stop the trastuzumab and use vinorelbine alone. Everybody should embrace this study, because it will help us answer this very, very important question.

DOCETAXEL-CAPECITABINE STUDY
In the docetaxel-capecitabine trial, concurrent use of docetaxel and capecitabine was better than single-agent docetaxel, and this surprised some people. The trial has been criticized, because not every patient who received docetaxel went on to receive second-line capecitabine. For the purist, trying to answer the question of sequential versus concurrent therapy, this trial doesn’t give us the exact answer. However, it is dramatic that there was a survival advantage in this trial, and we have to take that very seriously.
AMELIORATION OF CAPECITABINE-ASSOCIATED SIDE EFFECTS WITH DOSE REDUCTION
The side effects associated with capecitabine are minimal except for hand-foot syndrome. Occasional myelosuppression or diarrhea may occur, but I have not seen those often. Using 2,000 milligrams per meter squared per day for 14 days, hand-foot syndrome usually will occur in less than 25 percent of patients. If it does occur, I adjust the schedule and the dose a bit — sometimes I drop the dose to 1,500 milligrams per meter squared per day for 14 days, and I've tried one week on and one week off. We try to find a regimen that allows the patient to have the best quality of life.
Select References
Page 2 of 2
Previous Page
|
