| You
are here: Home: BCU 5|2003: Kathleen
I Pritchard, MD
Edited comments by Dr Pritchard
ATAC trial data update: 47-month follow-up
It shouldn’t have come as a surprise that anastrozole was
better than tamoxifen in the adjuvant setting, given what we know
from metastatic disease. This year’s updated results are
important — it’s reassuring to see the efficacy of
anastrozole holding up with little change in the toxicity profile.
I expect we’ll have survival data in a year or so, and if
that is significant, I think practice patterns will change quickly.
The unanswered questions that trouble me about anastrozole are:
Why are we giving it for five years? Is it because giving tamoxifen
for five years seemed to be best? Is three years better? Is seven
years better? These questions don’t suggest anastrozole is
an unacceptable alternative. We just seem to know less about it
and its long-term side effects.
I use anastrozole in the adjuvant setting primarily for patients
who can’t or won’t take tamoxifen, such as patients
with a history of thrombophlebitis. Physicians in Canada use anastrozole
as an alternative, rather than the standard, but patients are well-informed,
and they ask about it. When I discuss the possible complications
of tamoxifen, I tell my patients about anastrozole.
CALGB-9741: Dose-dense adjuvant chemotherapy
The data from CALGB-9741 is interesting. This trial had a two-by-two
design, and one could argue whether you should look at the four
individual blocks separately, or whether you can just interpret
it as a two-by-two trial. The investigators’ interpretation
is that the dose-dense approach is better than a standard approach.
We use doxorubicin/cyclophosphamide/paclitaxel as a standard
regimen in clinical trials and in practice, so we’d be interested
in knowing whether that combination is significantly better given
in a dose-dense fashion. I don’t know if this study is powered
to show that. The other question is whether the sequential therapy,
given in a dose-dense fashion, is as good as any of the other three
cells. If it is, that regimen may be the least toxic and that would
be interesting to know.
Some people believe that these particular regimens should now
be given in a dose-dense fashion. Some even believe that every
regimen should be given in a dose-dense manner, and I think that’s
wrong. It’s very intriguing that there may be a better approach,
but it’s too early for me to change my practice. I’d
like to see more data on the individual cells. Some data from other
investigators support dose density, but other results do not, so
it’s not clear to me whether we have enough data to support
this approach.
Canadian study of neoadjuvant CEF versus dose-intensified
EC
We are about to publish the results of a study comparing dose-intensive
EC to CEF in locally advanced breast cancer in the Journal of Clinical
Oncology. This was a large study of about 440 patients that we
conducted with the EORTC and the Swiss group. We found that the
dose-intensive EC was virtually the same as CEF. Patients in the
dose-intensive EC arm were given G-CSF, and we saw less febrile
neutropenia in that group but higher rates of thrombocytopenia
and anemia, so it is a bit of a trade-off.
This isn’t a real dose-dense study because the drugs in
the two arms aren’t the same. It’s more of a dose-intensive
regimen because we gave the epirubicin and cyclophosphamide in
half the time in the EC arm, and it was not superior. The curves
separated somewhat, but there was never a significant difference
between the two arms.
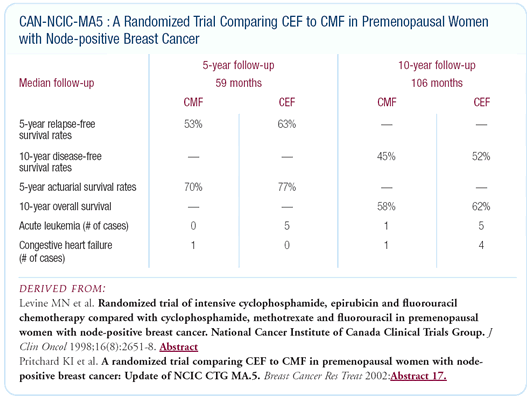
Canadian adjuvant trial comparing intensive
CEF versus standard CMF in premenopausal patients with node-positive
disease
At the 2002 San Antonio meeting, we presented data from our CMF
versus dose-intensive CEF trial with a nine-year median follow-up.
We designed the trial with a dose-intensive regimen to use as much
anthracycline as we could. We used epirubicin in that arm because
it is less cardiotoxic, and we matched the drug schedules in both
arms.
We published the data in 1998 with five-year median follow-up.
At that point, the data showed that the CEF was superior for disease-free
and overall survival, and it remains superior at this much longer
follow-up. We’ve looked at all the long-term side effects.
We saw five cases of acute leukemia in the CEF arm versus one case
in the CMF arm and four cases of congestive heart failure in the
CEF arm versus one case in the CMF arm. So while there are some
serious longterm toxicities, the rates are very low.

Next page
Page 1 of 2
|
