|
You
are here: Home: BCU Surgeons
Vol 2, Issue 2: Editor's
Note
 |
Editor’s Note
|
|
| Equipoise in Clinical
Trials |
The current practice of breast cancer medicine — and for
that matter, medical care in general — is dominated by the
need for research-based evidence to support clinical decision-making.
At the core of relevant clinical investigation is the randomized
trial, and one of the great challenges in designing these critical
studies is the requirement for randomization arms that physicians
can ethically and comfortably discuss with their patients. Researchers
often state that they must be “in equipoise with the options”:
all of the randomization arms are essentially equivalent, until
proven otherwise.
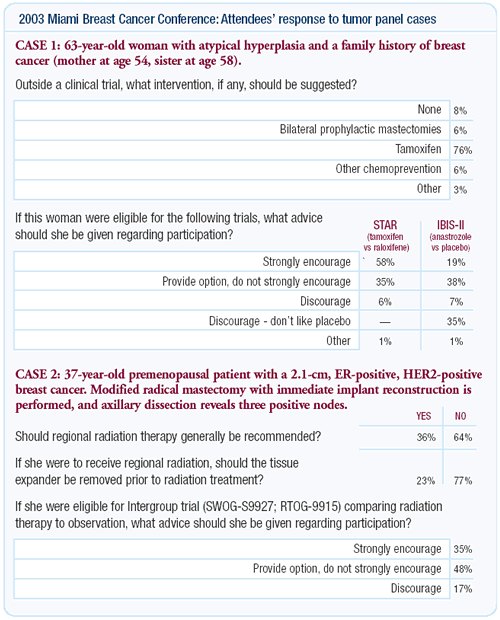
In this issue, we follow up on a series of tumor panel discussions
conducted at the 2003 Miami Breast Cancer Conference and interview
two faculty participants, Drs Monica Morrow and Jay Harris. One
of the fascinating aspects of the Miami meeting was the keypad
polling related to a number of current randomized trials. We focused
on some of the most controversial studies: RTOG-9915, which randomizes
women with one to three positive axillary nodes to either postmastectomy
radiation therapy or observation; American College of Surgeons
ACOS-Z-11, which randomizes women with positive sentinel node biopsies
to either axillary lymph node dissection or not; and IBIS-II, which
randomizes high-risk postmenopausal women to either the aromatase
inhibitor, anastrozole, or placebo.
During the Miami meeting, the attendees were asked what advice
they would give to their own patients about participation in these
studies. We were interested in this topic not only from a research
perspective, but also to gain another view of what people consider
standard of care. Both Drs Morrow and Harris support the postmastectomy
radiation trial, but acknowledge how difficult this randomization
is for both patients and physicians to accept. In this regard,
they reflected on another study with a challenging randomization — the
classic NSABP-B-06 trial — which randomized women to either
lumpectomy or mastectomy.
Dr Morrow encourages patients to enter the ACOS sentinel node
trial, but she has ethical reservations about IBIS-II because she
finds the placebo arm problematic in the presence of NSABP data
demonstrating a proven breast cancer risk reduction effect from
tamoxifen. Another interviewee, Dr Michael Baum, staunchly defends
IBIS-II because he feels the overall health benefit of tamoxifen
in women who are at high risk of developing breast cancer is marginal
or nonexistent.
The other speaker on this issue, Dr Bernard Fisher — who
is widely viewed as the “father of breast cancer clinical
research” — launched and championed NSABP-B-06 in a
furor of controversy several decades ago. Dr Fisher supports trials
evaluating aromatase inhibitors in women who are at high risk for
developing breast cancer, but he is more interested in “paradigm-breaking” studies
that will have a fundamental effect on our understanding of the
disease.
These debates are relevant not only to the research community,
but to all physicians providing care to breast cancer patients.
The current Phase III randomized trials will set new standards
for therapy over the next decade, and practitioners must be aware,
in advance, of the issues and controversies likely to evolve as
these trials mature. Perhaps of even greater relevance is that
discussions about ongoing studies provide perspectives on how the
most experienced breast cancer research leaders view the subtleties
of the risks and benefits of current available interventions. Ultimately,
the issue of equipoise with multiple treatment options is a daily
part of breast cancer medicine. Through this series, we attempt
to provide further perspectives on this challenging issue.
—Neil Love, MD
|
|
