| You are here: Home: BCU Surgeons 2004 Vol 3 Issue 2: Soonmyung Paik, MD
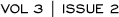

 |
Edited comments by
Soonmyung Paik, MD |
 |
Multigene prognostic test for women with node-negative, estrogen receptor-positive breast cancer treated with adjuvant tamoxifen in NSABP trials
Practicing medical oncologists have wanted a prognostic marker to help them select patients with node-negative, estrogen receptor-positive breast cancer who would be candidates for adjuvant chemotherapy. The NSABP developed a strategy to identify a strong, robust prognostic factor that would stratify such patients into low- and high-risk groups. With such a prognostic factor, the NSABP could tailor their clinical trials. For example, in patients with low-risk disease, we could focus our trials on optimizing local therapy with partial breast irradiation. On the other hand, in patients with high-risk disease, we could optimize approaches with chemotherapy or targeted therapy. Since we could not procure fresh tumor specimens, we had to develop a test that would work, reproducibly, using routinely processed paraffin blocks. About three years ago, the NSABP realized it was best not to develop this strategy in-house because eventually it needed to be available to the public. We decided to work with an industry partner, Genomic Health, who had a readily available technology that we identified as an extremely robust and reproducible methodology.
Cohort selection for multigene prognostic test development and validation
We wanted to make sure that we had two independent cohorts of similar patients one in which to develop the prognostic test and the other in which to validate it. In the NSABP's tissue bank of paraffin blocks, the most relevant cohort included patients with node-negative, estrogen receptor-positive breast cancer who were treated with adjuvant tamoxifen from two different trials (NSABP-B-20 and NSABP-B-14).
Gene selection for use in the prognostic test
Two hundred and fifty candidate genes were selected from the existing literature on the microarray analysis of breast cancer. Then, a real-time RT-PCR assay for paraffin blocks was developed for each gene. Two different study populations were then evaluated for the expression of the candidate genes, the results of which were presented at the 2003 ASCO meeting.
Obviously, not all 250 genes worked, and we ended up with 185 candidate genes to evaluate in the NSABP-B-20 cohort. Out of those, when correlated with more than 10 years of median follow-up, 41 genes were found to relate with clinical outcome on univariate analysis. On multivariate analysis, 12 genes remained highly significant. Then, we went back to two previous studies by Esteban and Cobleigh and determined whether any of those 41 genes were also prognostic in those cases, and we found about 12 common genes that were highly prognostic for all three completely different cohorts. Based on the findings from those three studies, we developed a prognostic algorithm with 16 cancer genes and five reference genes to be tested in the validation study.
Validation study for the multigene prognostic test
The validation study used the material from a prospective randomized clinical trial (NSABP-B-14). A single prognostic algorithm was validated in the NSABPB- 14 cohort. About 50 percent of the patients were identified as low risk (less than a 10 percent recurrence rate at 10 years), about 25 percent as intermediate risk, and the other 25 percent as high risk (Figure 3.1). We found a very significant difference between the risk groups. In a multivariate model, the patient’s age and tumor size were significant prognostic factors. However, based on the 21-gene algorithm, the recurrence score prevailed as the strongest prognostic factor.
Reporting the recurrence score
The recurrence score is a mathematical algorithm developed from the level of expression of the 16 cancer genes and five reference genes. We mathematically transformed the level of gene expression into a score that ranges from zero to 100. In the patients with a very good prognosis, about 82 percent have a score below 50. Actually, the recurrence score is a continuous variable without a cutoff. A linear relationship exists between a recurrence score of up to 50 and the 10- year cumulative recurrence rate. The best use of this recurrence score algorithmis as a continuous variable, rather than grouping the patients together. When a patient receives a report, it consists of a numerical score with an estimated 10-year recurrence rate, plus or minus a very narrow confidence interval.
Quality control for HER2 testing
When the NSABP designed the B-31 adjuvant trastuzumab trial, we were very reluctant to require central testing for HER2. I always believed that it was not possible for a pathologist to misclassify patients with IHC 3+ overexpression, and the entry criteria for the study required patients’ tumors to be IHC 3+. However, we built a safeguard into the protocol such that we would perform central testing in the initial 100 patients entered into the study.
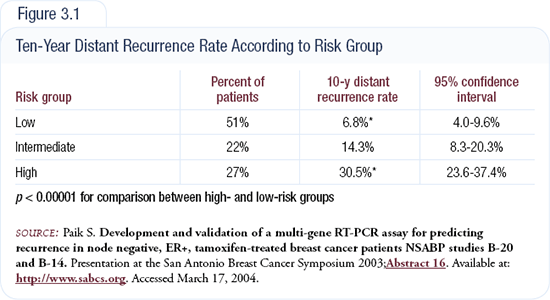
HER2 status was measured by both IHC and FISH, so HER2-negative tumors were truly negative. We were shocked, because the false-positive rate was 18 percent (Figure 3.2). The Intergroup trial demonstrated essentially the same finding, and these results were a big “wake-up call” for the community.
Based on the false-positive rate, we revised the protocol so that patients had to be tested by an approved laboratory, which included those performing over 100 tests per month or those performing fewer tests but demonstrating a concordance rate between IHC and FISH of over 95 percent. The end result was a dramatic improvement in the quality of test results; the false-positive rate dropped from 18 percent to three percent (Figure 3.3).
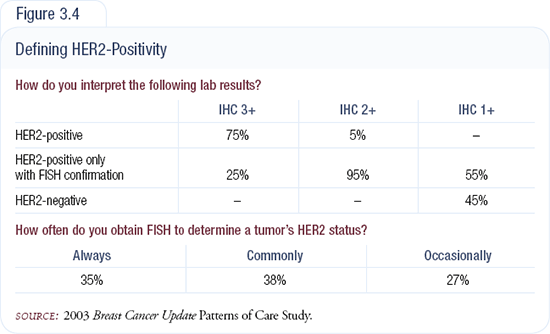
Clinically, oncologists should demand to know the concordance rate between IHC and FISH in the laboratories they utilize. Over 95 percent of patients with IHC 3+ tumors should have been validated as FISH-amplified (Figure 3.4). Oncologists should also examine the concordance rates between IHC 0 and 1+ and FISH, because false-negative results have extremely important clinical implications. The College of American Pathologists published a recommended format for the HER2 IHC report that clearly indicates this information should be provided by laboratories.

Select publications
|
| Dr Paik is the Director of the NSABP Division of Pathology in Pittsburgh, Pennsylvania |
|
