| You are here: Home: BCU Surgeons 2004 Vol 3 Issue 4 : Frank A Vicini, MD
Frank A Vicini, MD |
EDITED COMMENTS |
 Interstitial brachytherapy Interstitial brachytherapy
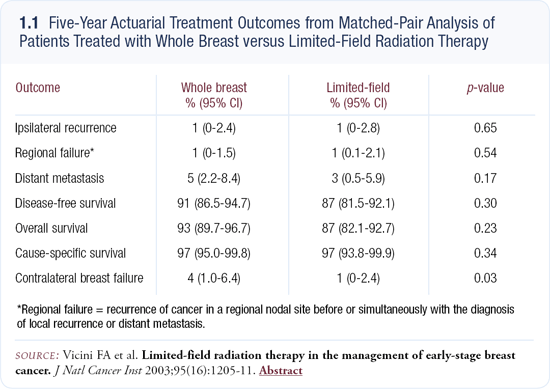
At William Beaumont Hospital, we matched 199 patients treated with interstitial brachytherapy with 199 patients who received conventional external beam radiotherapy. With a median follow-up for surviving patients of 65 months, we found the endpoints to be equivalent, including local control rates, regional failure rates and cause-specific survival (1.1). In the past 10 years of published data, the collective experience with interstitial brachytherapy consists of approximately 500 to 600 patients, compared to tens of thousands of women treated with whole breast radiation therapy. With brachytherapy, we have only small numbers of highly selected patients treated at single institutions. We don’t really know what the efficacy will be in larger patient populations with less restrictive criteria.
Partial breast irradiation
One of the advantages of partial breast irradiation (PBI) is that it can be completed quickly before systemic therapy is initiated. Our surgeons are progressive in this field. William Beaumont is one of the few institutions that offers interstitial brachytherapy, MammoSite® and conformal external beam radiation therapy. Each technique has its advantages and none of them are applicable to all clinical scenarios. Treatment must be individualized based on factors such as the patient’s access to a radiation facility and the location of the lesion within the breast.
At our institution, of the patients who receive PBI, approximately 60 percent are treated with the MammoSite®, 30 percent with conformal external beam radiotherapy and a small percentage with interstitial brachytherapy. Some physicians question whether it’s worthwhile to study PBI given the high efficacy and low toxicity achieved with breast conservation using whole breast radiation therapy. However, in the United States a large proportion of women do not undergo breast-conserving therapy. A recent study showed that the distance to a radiation facility still factors into a woman’s decision-making. In addition, some people fear radiation. Reducing the amount of time required and the amount of toxicity associated with radiation therapy may increase the rate of breast conservation. I believe that an additional 10 to 20 percent of women making this decision would select breast-conserving therapy if PBI was an option.
Proposed NSABP-RTOG trial comparing whole breast radiation versus PBI
The NSABP and RTOG plan to conduct a joint study that will randomly assign 3,000 patients to conventional whole breast radiation therapy versus one of three PBI techniques — interstitial brachytherapy, MammoSite®, or 3-D conformal external beam radiation. The eligibility will be broad, with no age restrictions. It will include patients with DCIS, infiltrating lobular histology and up to three positive nodes. Patients with four or more positive nodes will be excluded because they are candidates for regional nodal radiation therapy. Randomization will occur after surgery to ensure the pathology criteria are met.
Partial breast irradiation for DCIS
The American Brachytherapy Society (ABS) has developed recommendations for the off-protocol use of brachytherapy. Based on the data currently available, the ideal patients are those with tumors less than three centimeters, negative lymph nodes, negative margins and no extensive intraductal component. They exclude patients with DCIS because only a small number of such patients have been treated with this technique. I suspect that this will change in the next few years. Considering the applications for PBI, I believe patients with DCIS are ideal for testing this concept because the issue of a survival disadvantage is no longer arguable. The only difference between whole breast irradiation and PBI is that the latter targets the tissues that most likely need it. I consider PBI a reasonable compromise between no radiation and six and a half weeks of radiation, which is probably overkill in the majority of these patients.
MammoSite® placement
The MammoSite® can be placed either during or after surgery. There are more than 1,000 patients on the MammoSite® registry, and the device was placed intraoperatively in approximately 50 percent. However, it appears the more experienced institutions prefer to place it postoperatively. I prefer the postoperative, closed-cavity technique because it allows me to obtain the pathology results and determine the technical feasibility of any PBI procedure before I discuss it with the patient. It’s distressing for a patient to learn she is not a candidate for PBI after a device or needles have been placed.
A patient may be ineligible based on pathologic factors such as positive margins, large tumors, lobular histology, DCIS or positive nodes. A CAT scan is performed postoperatively to rule out technical difficulties. In placing the MammoSite® it is important to keep the surface of the balloon at least five to seven millimeters away from the skin surface to avoid excessive radiation to the skin. In the registry data, 94 percent of patients treated with adequate spacing achieved good to excellent cosmetic results. We only have one to two years of follow-up, but early cosmetic results generally predict late results and I doubt we’ll see any unusual late cosmetic effects.
Off-protocol use of the MammoSite®
The MammoSite® is easier for surgeons to use and patients to accept, but some physicians are concerned that it’s being disseminated to the community before it’s been fully tested in randomized trials. Many argue that we cannot extrapolate the interstitial experience to the MammoSite®, and experience with the interstitial approach itself is very limited with only five years of follow-up. Some worry that because it’s hyperfractionated radiation, we’ll encounter very late deterioration in cosmetic results not seen in the five-year data.
While I favor enrolling patients in randomized trials, data from the current trials won’t be mature and analyzed for at least eight years after accrual is completed. I was involved in writing the ABS recommendations and we stated that with informed consent and in selected patients, it is reasonable to offer the MammoSite® off protocol. Most new concepts in medicine are not proven in Phase III trials before they’re used in clinical practice, as seen with sentinel node biopsy. I believe it’s more reasonable to give recommendations on the optimal use of this technique than to blatantly oppose its use off protocol.
Select Publications
 |
Dr Vicini is Chief of Oncology Services in Oncology Services Administration at William Beaumont Hospital in Royal Oak, Michigan. |
|
