| You are here: Home: BCU Nurses 2004 Vol 2 Issue 1: Excerpts from the Audio Program
 |
Excerpts from the Audio Program: |
|
 |
Determining the Risk of Recurrence and Impact of Adjuvant Systemic Therapy
We evaluate tumor size, grade, node status and whether hormone receptors and HER-2 receptors are present. With all of those characteristics, we estimate prognosis and the risk for recurrence. Based on extensive data from many clinical trials, we can also determine the potential benefit of a number of possible adjuvant treatments and whether the patient might be eligible for a clinical trial.
Often, I have a patient with an excellent prognosis after local therapy and endocrine treatment and the additional benefit that patient will derive from chemotherapy might be as low as one percent. In that case, the patient has to decide whether the risks and toxicities of going through chemotherapy are really worth the one percent extra benefit. I don’t think that is for me to decide; it’s for the patient to decide. My role as a physician is to inform the patient and give her the tools she needs to make the right decision. It amazes me how often these women will choose to receive chemotherapy for such a small benefit.
— Sandra Franco, MD
Breast cancer patients don’t ever want to look back and say, “I should have been treated. Did I lose a chance to be cured by not moving forward with a therapy that’s been recommended?” In the adjuvant setting, women often have a very difficult time with the decision. The most important message we provide to these women is, “In the adjuvant setting, this is our opportunity to cure your disease. And once we are beyond this setting and dealing with metastases, we can certainly control your disease and you can live with good quality of life if things go well, but if we don’t take the opportunities that exist in the adjuvant setting, we don’t have another opportunity.” And that seems to hit home.
— Cynthia Frankel, RN
Mechanism of Action of Endocrine Therapies
Tamoxifen is a selective estrogen-receptor modulator that binds to the estrogen receptor and causes inhibition of signal transduction by blocking the receptor. The estrogen receptor is a complicated structure that has two parts. Tamoxifen will activate one part of the receptor and de-activate the other. That is why it is called a modulator.
Fulvestrant, on the other hand, will de-activate both parts of the estrogen receptor, causing full blockage of signal transduction and resulting in disappearance of the estrogen receptor.
By contrast, aromatase inhibitors work away from the cancer cell by blocking aromatase enzymes in the breast, liver and fat. Aromatase converts two androgenic hormones produced in the adrenal gland — androstenedione and DHEA — into two estrogenic hormones —estradiol and estrone. Even though levels are much lower, estrogen production continues in postmenopausal women. Thus aromatase inhibitors can be used to essentially block the conversion of androgens into estrogens.
— Sandra Franco, MD
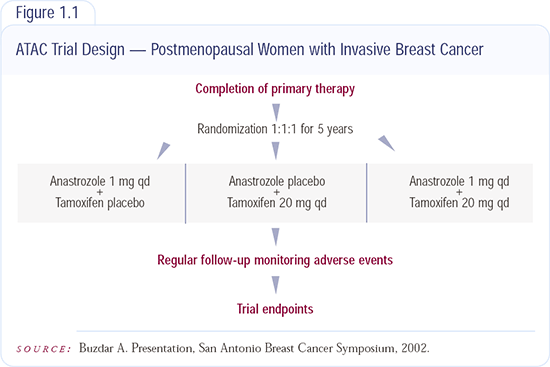
ATAC Adjuvant Trial Results
There are over 9,000 patients from all over the world in this study, with just over 3,000 patients in each arm. The headline news is that it looks as if there is something after tamoxifen — there is a significant advantage to anastrozole compared to tamoxifen. The real surprise is that the combination of anastrozole and tamoxifen looks no different than tamoxifen alone.
At this point, my preferred therapy for the postmenopausal woman with receptor-positive breast cancer is anastrozole unless contraindicated — for example, in women with high risk of osteoporosis or with osteoporosis. If a clinician is concerned about a loss of bone mineral density, it’s something that could be monitored. You don’t withhold chemotherapy because you’re worried about white cell count — you give it but you closely monitor white cell count. Osteopenia is not a crisis like neutropenia can be, and by doing a bone mineral density at entry and then intervening with a bisphosphonate when necessary, say if bone mineral density is starting to fall, it can be managed. So the one adverse effect favoring tamoxifen over anastrozole, I think, can be managed.
— Michael Baum, ChM, FRCS
Implications of the ATAC Trial in Clinical Practice
The results of the ATAC trial are quite compelling. Even if you assume for the sake of argument that the disease-free survival curves will come together with further follow up, the safety profile of anastrozole is still clearly better than that of tamoxifen. I cannot prevent endometrial cancer short of removing the uterus, but I can prevent or treat osteoporosis and fractures.
Since the safety profile of anastrozole is better than that of tamoxifen and it is therapeutically superior, I have a problem not offering anastrozole to my postmenopausal patients — not as a neutral choice but as a better choice. I discuss with my patients the enormous amount of clinical experience we have with tamoxifen, but if my sister were postmenopausal and developed breast cancer today, I would certainly recommend anastrozole as opposed to tamoxifen.
— Gabriel N Hortobagyi, MD
After the ATAC trial results were initially presented, I began speaking with my patients about the results. I told them that the data were still early, and we discussed the risk-benefit profile and quality-of-life issues. Then they made the decision to receive anastrozole or tamoxifen.
There are some women who are so concerned about the potential for osteoporosis with anastrozole, or who already are suffering from arthritic symptoms, that they will say, “If those side effects are predominant with anastrozole, then I’d prefer to go with tamoxifen.” On the other hand, women who are deathly afraid of the uterine cancer risk and blood clots may choose to go with anastrozole. At this time, both of these are still very viable options.
— Charles L Vogel, MD, FACP
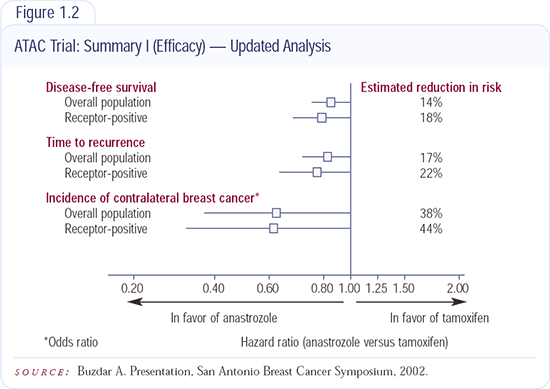
Safety Profiles in the ATAC Trial
One of the most exciting parts of the ATAC trial is the safety profile of anastrozole. There was a highly significant reduction in the incidence of hot flashes, vaginal discharge and vaginal bleeding. This reduction in vaginal bleeding is significant because it will cut down the number of women referred to gynecologists to rule out endometrial cancer.
Perhaps even more important is the significant reduction in the anastrozole arm in life-threatening events such as strokes, cerebrovascular accidents and thromboembolic events.
In terms of side effects, about 8 percent of women receiving anastrozole complain about arthralgias. There is also a numerically modest but highly significant excess fracture rate in the anastrozole arm. Apart from bone mineral density — which I think we can handle if we anticipate it — the safety profile strongly favors anastrozole over tamoxifen.
— Michael Baum, ChM, FRCS
Endometrial Cancer and Tamoxifen
I think women understand that even though we use adjuvant therapies to attempt to cure their disease, there is a risk of some of them causing other cancers. I don’t know what could be scarier and I think that is the reason many patients who are on tamoxifen focus on endometrial cancer even though there is just a one percent risk.
— Cynthia Frankel, RN
Implications of Canadian and Italian Trials of Sequencing of Tamoxifen and Aromatase Inhibitors
A recently presented Italian trial has shown a significant benefit for patients switching to anastrozole following two or three years of tamoxifen. These data go along with data from a Canadian trial showing a similar result for starting an aromatase inhibitor after five years of tamoxifen. There is still a lot about this that is not known; however, I think these new data should be discussed with women who are currently being treated with tamoxifen or who have completed five years of tamoxifen so that they can make their own decisions about switching to an aromatase inhibitor.
— Sandra Franco, MD
Providing Patients with Treatment Options and Recommendations
One of our major goals is to fully educate our patients by giving them relevant, accurate and complete information, so that they understand their prognosis, treatment options and the benefit-to-risk ratio they will face with each of those options. But we can’t stop there. We also need to make a recommendation after that education. Obviously this recommendation will incorporate our biases and prejudices, but we are better qualified — even with those biases and prejudices — than a patient who just had “oncology 101” during the previous 20 to 30 minutes.
Over the past 30 years in medicine we have moved from a paternalistic approach to the other extreme. Many of my colleagues try to be so neutral that they do not make a recommendation. The burden of decision making has been removed completely from the physician, who is best qualified to make that choice or recommendation, to the patient, who sometimes is but most of the times is not in the best position to make that choice without guidance.
I understand and agree that patients need to have autonomy. We clearly have the obligation to inform them fully, but I think we need to go beyond that. We have to get to know our patients and understand their motivations, their understanding of risks and benefits, their definition of therapeutic gain and their level of acceptance of risks and side effects. As physicians, we need to help them make a decision. To abrogate that responsibility is an unfortunate — and I hope temporary — trend in the medical profession.
— Gabriel N Hortobagyi, MD
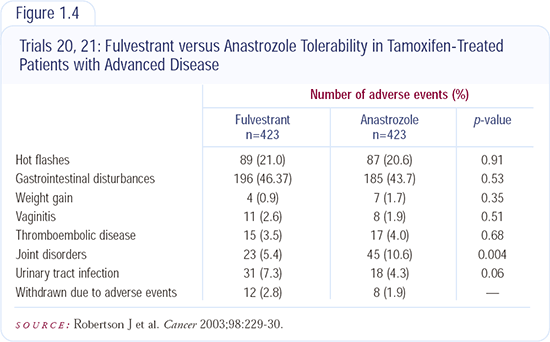
Tolerability of Fulvestrant
Injection site reactions and hot flashes are the only side effects that I’ve observed in patients receiving fulvestrant. There may be something about the administration technique for fulvestrant that can affect the pain that is infrequently experienced. If the injection is inadvertently given subcutaneously into fat, it’s more painful than if it’s given intramuscularly. It may be that many of the women who have pain with the injection are not actually receiving true intramuscular injections; this is more likely to occur in women who are obese.
— Robert W Carlson, MD
We were involved in the initial North American trial of fulvestrant versus anastrozole in women with ER-positive tumors. I personally administered many of the injections to patients in the trial and they were tolerated very well. No patient dropped out of the trial because of the injections. The bottom line with fulvestrant is that it is an oily substance and it takes a good minute to give the injection. You need to take your time.
Sometimes a little bit of seepage can cause a rash in women receiving their first injections. When we give the injections deep in the muscle, hold pressure and wipe off that area to remove the seepage, no injection reactions are noted.
— Sharon Carrasco, RN, MSN, OCN
Sequencing Hormonal Agents in Postmenopausal Women
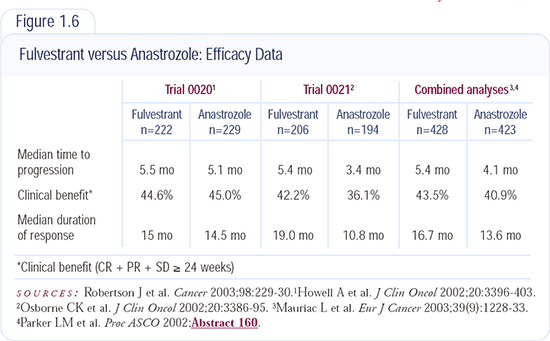
In a postmenopausal woman whose disease relapses on adjuvant tamoxifen, I would use fulvestrant because I’ve seen some very long remissions with it. I will use an aromatase inhibitor later because data indicate that patients with disease that progresses on fulvestrant can still respond to other endocrine treatments (e.g., aromatase inhibitors and megestrol acetate).
A couple of reports have evaluated the response to fulvestrant in patients who have received an aromatase inhibitor. A fairly small Swiss study reported that about one-third of patients derived clinical benefit from fulvestrant after treatment with tamoxifen or an aromatase inhibitor. A compassionate-use study reported at ASCO 2003 reported about 60 patients with fulvestrant as second-, third- or fourth-line therapy. Fulvestrant had a more than 50 percent clinical benefit rate in those patients.
— Stephen E Jones, MD
Women with breast cancer who fail on tamoxifen can clearly respond to fulvestrant, and the rate of response is equivalent to that seen with anastrozole. Also, in women with disease that has failed anastrozole who are then crossed over to fulvestrant, the rate of clinical benefit is substantial and in the range of approximately 40 percent. Patients who are crossed over from fulvestrant to aromatase inhibitors also show response rates of approximately 40 percent.
Surprisingly, the magnitude of benefit from fulvestrant does not predict whether the cancer will respond to a subsequent hormonal maneuver. One rule of thumb in the past has been that the magnitude and duration of response to the most recent hormonal therapy predicts for the likelihood of response for subsequent hormonal therapies. A small retrospective study suggests that may not be the case with fulvestrant.
— Robert W Carlson, MD
We know that fulvestrant is at least as efficacious as the aromatase inhibitors, but I really don’t know where I would sequence it because the aromatase inhibitors are pills. I think fulvestrant is and will continue to be most useful for patients on fixed incomes who can’t afford the aromatase inhibitors or those for whom there are concerns about their reliability in taking oral medications. Women in those situations can be treated quite nicely with fulvestrant, and we have data indicating that it is probably at least equivalent.
— Charles L Vogel, MD, FACP
Fulvestrant’s Mechanism of Action
Fulvestrant binds with the estrogen receptor monomer in the cytoplasm and prevents the dimerization of the estrogen receptor, which is required for exertion of its maximal activity. Lack of estrogen receptor dimerization results in accelerated degradation of the ER-fulvestrant complex. Ultimately, there is a loss of estrogen receptors within the cells.
The estrogen receptor is continually regenerated, so continued exposure to fulvestrant is required. After fulvestrant is discontinued, the estrogen receptor will, with time, reappear in cells. The fact that we see subsequent hormonal responses is convincing biological or clinical evidence that the estrogen receptors do reappear.
— Robert W Carlson, MD
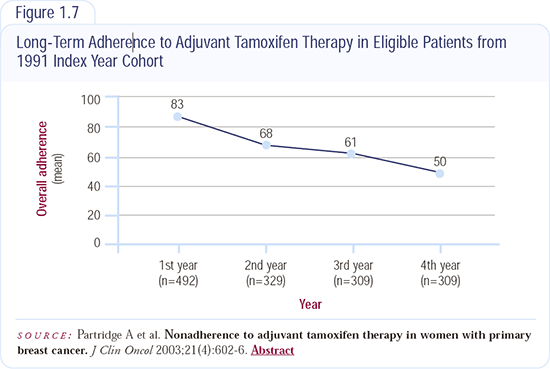
Compliance with Oral Therapy
Compliance is definitely an overlooked problem. You would think that a woman with symptomatic metastatic disease who is participating in a clinical trial would be constantly reminded of her disease enough to take her treatment, but I have had patients come in and say, “I forgot to take my study drug.” Now think about someone with very stable metastatic disease that comes in every month or every three months. There is no doubt in my mind that compliance is an issue.
— Sandra Franco, MD
There is a commercial that says “it’s not hard to remember, it’s just easy to forget,” and I think women like the fact that they can come in, receive their therapy and not have to worry about taking their medication every day. I think fulvestrant probably increases compliance and decreases anxiety.
When I first started working with women with breast cancer, most of them sort of gave up their lives and spent the rest of their time just getting their ducks in order. With breast cancer today, women are raising families, working and making their place in society. Many of them can barely make time for their treatments, so I think compliance is definitely an issue.
—Cynthia Frankel, RN
|
