| You are here: Home: BCU 5|2002: Editor's Note
Editor's Note
Predicting the Results of Clinical Trials
Every five years, breast cancer research leaders make a pilgrimage to Oxford, where Sir Richard Peto presents the most recent results from an international meta-analysis of virtually every known randomized clinical trial in early breast cancer. This massive undertaking painstakingly incorporates a case-by-case “data cleaning” of each individual trial. In 1990, I was privileged to attend the second trialists’ meeting. At breakfast that day, rumors circulated that the soon-to-be-knighted statistician and his team worked all night to have the data ready on time. Looking a bit haggard, but with a bemused expression, Peto appeared and began his day-long presentation.
Always one to push researchers to think creatively, Peto distributed a survey prior to the meeting asking the attendees to predict the results of the meta-analysis. At each critical presentation point, he first reviewed the trialists’ predictions followed by the actual findings, many of which were very different than expected. In particular, most of the researchers predicted that — with five more years of follow-up — the differences in the Kaplan Meier plots for disease-free and overall survival for adjuvant tamoxifen would narrow. With a broad smile, Peto disproved these predictions, and indeed, with the additional follow-up, the curves for adjuvant tamoxifen compared to placebo were even farther apart — a trend that persisted in subsequent overviews.
Seasoned clinical researchers know the hazards of predicting randomized trial results, and many cite the lofty, but unfulfilled, expectations in the early 1990s for high-dose chemotherapy with stem cell support. In the accompanying audio program, George Sledge reminded me of this lesson and cautioned clinicians about moving too fast to adopt trastuzumab as adjuvant therapy, until randomized trial data are available.
Another speaker on this program, Tony Howell, noted that many clinicians and researchers assumed that the anastrozole-tamoxifen arm of the ATAC trial would yield the greatest benefit. This prediction was invalidated by the initial ATAC trial results, which demonstrated anastrozole’s superiority.
The entire culture of cancer therapy is now focused on evidence-based medicine, and Craig Henderson — a champion of this philosophy in breast cancer — verbalizes in the enclosed interview his struggle to make sense of the age-based efficacy of chemotherapy demonstrated in the Overview. In the 1980s, the triumvirate of Craig Henderson, Richard Peto and Michael Baum played a critical role in educating the research community and practicing clinicians about the need for trials with an adequate numbers of events. Simultaneously, sentinel figures like Bernie Fisher and Charles Coltman provided the leadership within cooperative groups to make this happen.
Echoing the challenge of advancing the field through large well-designed studies, Terry Mamounas’ interview traces the background and design of the current and planned NSABP clinical trials. Dr Mamounas discusses the encouraging results of the capecitabine-docetaxel trial by O’Shaughnessy et al demonstrating a survival advantage in metastatic disease compared to docetaxel alone. These results led the NSABP to design two clinical trials that incorporate this combination — a neoadjuvant study comparing AC-> docetaxel to AC-> capecitabine/docetaxel and a study of capecitabine/docetaxel in women with a local recurrence of breast cancer.
Shortly after my interview with Dr Mamounas, a relevant and important new data set became available, namely the initial results of BCIRG 001. These findings, presented at the May 2002 ASCO meeting in Orlando by Dr Jean-Marc Nabholtz, demonstrated an advantage in node-positive patients for TAC (docetaxel, doxorubicin, cyclophosphamide) in the adjuvant setting (see table on page 5). Prior to the ASCO meeting, we surveyed 20 medical oncologists to determine their predictions of this study’s results and how they integrated clinical trial information into their practices. Whether the very existence of an ongoing randomized study encourages physicians to utilize the experimental arms in a nonprotocol setting is of particular interest.
It would be interesting to compare the current practices of clinicians, in this regard, to those of 10 or 15 years ago. My guess is that lessons learned from our previous failures to predict clinical trial results have led to a wave of conservatism, and experienced practitioners now are very cautious about changing their practices until clear-cut advantages are demonstrated in well-designed and conducted randomized studies.
— Neil Love, MD
Select Publications
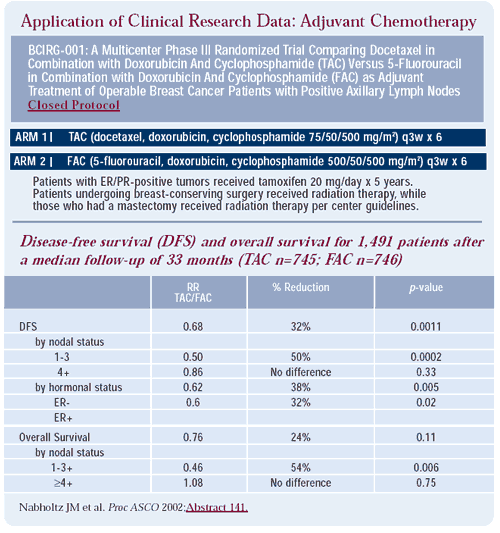
Comment: Although adjuvant trials often initially demonstrate benefits in higher-risk patients, where there are more events, BCIRG 001 demonstrated a significant disease-free and overall survival benefit for TAC in women with 1-3 positive nodes, but not in women with four or more positive nodes. In contrast to retrospective analyses from the Intergroup and NSABP trials with paclitaxel, TAC improved disease-free survival regardless of the hormone receptor status.
Select Publications
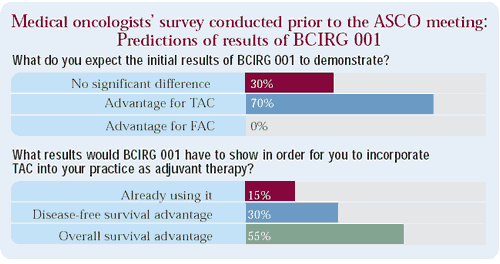
Comment: While most oncologists predicted that the TAC arm of this study would be superior, relatively few were utilizing this combination prior to these results being presented. An overall survival benefit is more highly valued than a disease-free survival benefit in terms of changing clinical practice.
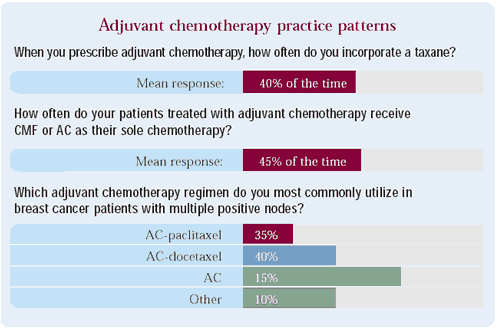
Comment: Even prior to the BCIRG 001 presentation, clinicians were commonly utilizing taxanes as adjuvant therapy, particularly in node-positive patients. Interestingly, most of the available randomized clinical trial data on this subject has come from meeting presentations (ASCO, 2000 NIH Consensus Conference) as opposed to publications in peer-reviewed medical journals.
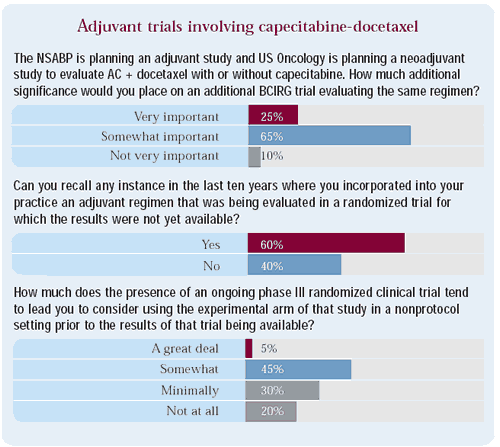
Comment: The accumulating evidence suggesting a modest advantage for the addition of taxanes to adjuvant therapy is leading cooperative research groups to consider assessing capecitabine in combination with docetaxel. While two major US cooperative groups are considering trials evaluating this combination in the adjuvant and neoadjuvant setting, clinicians are also interested in other studies evaluating this question.
While more than half of the oncologists surveyed indicated that they have used an experimental arm from an adjuvant randomized trial as nonprotocol therapy, only a small number state that the presence of a randomized study significantly affects their likelihood to utilize an experimental approach prior to its demonstrating an advantage.
|
