| You are here: Home: BCU 5|2002: I Craig Henderson, MD
 |
 |
 |
 |
 |
I Craig Henderson, MD |
 |
 |
Adjunct Professor, Hematology/Oncology
University of California San Francisco
Member, Cancer and Leukemia Group B (CALGB)
Steering Committee Member, Bay Area Breast Cancer Translational Research Program (NIH Specialized Program of Research Excellence, SPORE)
Former Chairperson, Early Breast Cancer Trialists' Collaborative Group |
|
 |
 |
 |
Edited comments by Dr Henderson
Implications of ATAC for clinical practice
The most important and exciting data to emerge in breast cancer clinical research this year were the ATAC trial results, which demonstrated a dramatic disease-free survival advantage for anastrozole over tamoxifen as well as a more favorable toxicity profile. These data are from a very large, credible trial, and clearly, I am obligated to discuss these results with my patients. However, I am still uncertain how these data will affect my practice.
There is not yet enough follow-up to determine whether anastrozole will improve survival, and we need more information about its effect on bone mineral density. Trevor Powles' data demonstrating that a bisphosphonate reduces the incidence of bone metastases is compelling. Therefore, one option would be to give a bisphosphonate concurrently with anastrozole. In my practice I still tend to recommend tamoxifen, but I am much more comfortable switching patients to anastrozole if they cannot tolerate tamoxifen.
Management of postmenopausal patients with ER-positive breast cancers
For the 60- to 70-year-old, ER-positive patient I would discuss the very modest benefits of chemotherapy. If a patient chooses chemotherapy, then I would support that as a reasonable option but would not recommend it. These women should receive hormonal therapy.
Even patients who have tumors with a few percent, weakly staining estrogen receptors will have surprisingly large responses to hormonal therapy. If a tumor is predominantly hormone-sensitive, then the added benefit from chemotherapy is going to be very small. Additionally, we know that the older the woman, the smaller the benefit from chemotherapy. In postmenopausal women, the effect from chemotherapy on survival is about one-half to onethird of the effect in premenopausal women. If you take the older women who have ER-positive tumors, the effects from chemotherapy are even less.
The diminishing effect of chemotherapy in older women
We know with certainty that as women get older they derive less benefit from chemotherapy. In younger women, the reduction in the odds of death are slightly higher than 30%, but in 60- to 70-year-old women the reduction is only about 9%. Interestingly, the reverse is seen with tamoxifen — with older women receiving greater benefit than younger patients.
In the 1980s, my initial reaction to this data was that it reflected the effect of chemotherapy on the ovaries. However, Richard Peto analyzed the relationship between menopause and the effects of both chemotherapy and tamoxifen. There is a highly significant linear effect with age and response to chemotherapy and tamoxifen but not with menopause. That did not make sense to me, but it is definitely a real phenomenon, and for 15 years I have been trying to figure it out.
Postmenopausal ovarian function
There is a misconception that once a woman stops menstruating, the ovaries immediately cease to function. That is too simplistic. Ovarian function continues for some time after the onset of amenorrhea, and it is reflected in circulating estrogen levels. One of the best studies to evaluate this issue indicated that, on average, the ovary continues to produce estrogen for four years after cessation of menses and up to ten years in some women. Additionally, more testosterone and androstenedione are being produced by the ovaries in older women. In fact, probably one-half of the androgens in a postmenopausal woman are produced by the ovary, and the other half come from the adrenal gland.
There are data to suggest that chemotherapy reduces both estrogen and androgen levels even in postmenopausal women. Another little pearl that people have forgotten comes from the work of Nissen-Meyer, who conducted one of the earliest oophorectomy trials back in the 1950s, which included postmenopausal women. He demonstrated that postmenopausal women responded to oophorectomy, but only the older postmenopausal patients. The ones who are just a few years after menopause do not respond.
A group of surgeons at the University of Oregon did an oophorectomy series in the 1980s and produced exactly the same results. In the early 1980s, I published a paper demonstrating that premenopausal and very postmenopausal women received the greatest benefit from various hormone therapies, but there was a blip in the perimenopausal years where no type of endocrine therapy worked very well. So, the ovary is not unimportant in postmenopausal women.
Effect of adjuvant chemotherapy in ER-positive patients
In the past year, I have been trying to understand why ER-positive patients did not benefit from the addition of paclitaxel to AC x 4 in the Intergroup adjuvant trial 0148 (CALGB 9344). My initial reaction was that because these patients received tamoxifen, there was little additional effect to be gained from chemotherapy. I evaluated this hypothesis by examining all the trials in the Overview that gave one, two and five years of tamoxifen plus or minus chemotherapy. If my hypothesis was correct, then adjuvant chemotherapy would have demonstrated greater benefit in those receiving a shorter compared to a longer duration of tamoxifen. That did not prove to be the case.
Currently, my hypothesis is that in both pre- and postmenopausal, ER-positive patients the effect of adjuvant chemotherapy is mediated through the ovary. AC x 4 does not cause a lot of amenorrhea, and I suspect that the taxanes do not give as much ovarian suppression as daily, oral cyclophosphamide. It may be that neither AC x 4 nor paclitaxel x 4 represent optimal treatment if you want to achieve ovarian suppression.
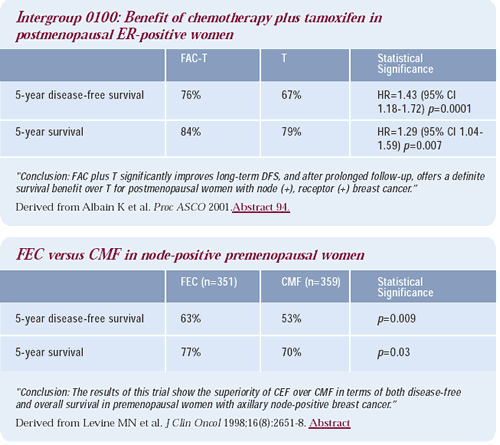
For premenopausal patients, there is an abundance of data demonstrating that chemotherapy regimens that induce amenorrhea — such as the classic Bonadonna CMF regimen — are among the most effective therapies. The Canadian adjuvant FEC trial produced dramatic benefits, and it also used daily, oral cyclophosphamide for 14 days. At the 2001 American Society of Clinical Oncology meeting, Kathy Albain presented the results from an important Intergroup study that randomized ER-positive women — who were no longer menstruating — to tamoxifen plus or minus classic FAC, utilizing that same dosing schedule of cyclophosphamide. FAC-T resulted in a significant diseasefree and overall survival advantage compared to tamoxifen alone.
Select Publications
|
