| You are here: Home: BCU Surgeons |2002: Patrick Borgen, MD
Edited comments by Dr Borgen
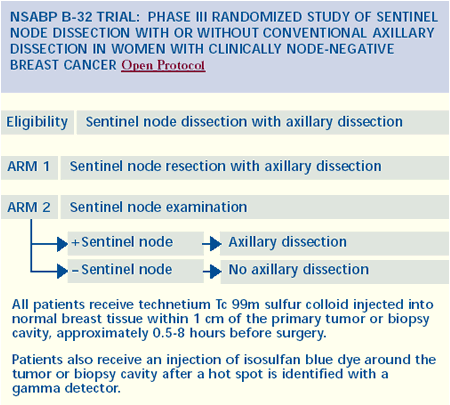
Sentinel node biopsy: The standard of
care?
In experienced hands, sentinel node biopsy is absolutely a standard
of care. The evidence is overwhelming. There are over 50 institutional
series of 6,000 patients who had back-up axillary dissections. Sentinel
node biopsy is actually more accurate than providing the pathologist
20 lymph nodes from an axillary dissection.
The question should not be whether sentinel node biopsy is an
accepted standard of care, but rather, does each surgeon know how
to do it, and is he or she comfortable accepting a sentinel lymph
node biopsy result?
Patient selection is important — sentinel node biopsy is
not for everyone. For example, if the tumor is very high in the
tail of the axilla, sentinel node mapping can be problematic. Other
examples of women who are not good candidates for sentinel node
biopsy are patients with two cancers in one breast, clinically palpable
nodes and those who have received neoadjuvant chemotherapy.
Parenchymal versus intradermal tracer
injections for sentinel node biopsy
We started doing sentinel node biopsy with parenchymal injections
of tracer in 1995, and in 1998 we began studying the intradermal
injection. This became our standard of care in late 1999 because
of the advantages to the intradermal injection.
First, the skin lymphatics drain much faster than the parenchymal
lymphatics. Second, we can use a physician extender to inject the
tracer — the surgeon doesn’t have to go to nuclear medicine
to do the injection. And third, we can use two-thirds less radioactivity
when injecting into the skin. The radioactivity in the lymph node
is lower, and there is less radiation exposure to pathologists handling
these nodes.
This intradermal technique is now being done widely. Kelly McMasters*
from Louisville studied surgeons and found that the learning curve
was far easier, and the success rate was much higher with intradermal
injections of tracer than it was with intraparenchymal injections.
*McMasters KM. Ann Surg 2001;233(5):676-87.
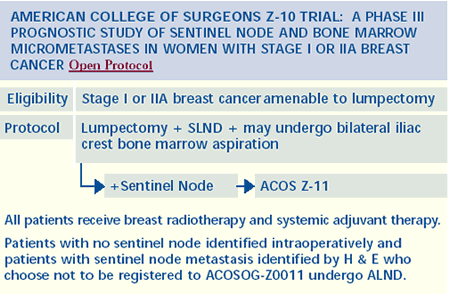
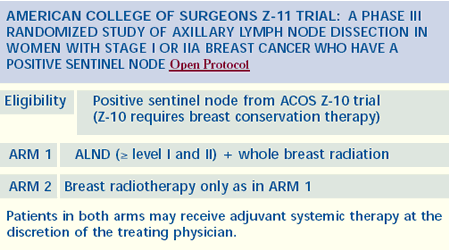
Intraoperative radiation therapy
We have an active research protocol looking at intraoperative
radiation therapy to the quadrant of the breast with the cancer.
This would be an enormous advantage if we can prove that it is as
safe and efficacious, over six weeks of external beam radiation
therapy. It is one-third as expensive, completed in 30 minutes rather
than six weeks, and patients can start systemic therapy immediately.
We are very enthusiastic about the idea of jointly doing surgery
and radiation therapy.
Veronesi’s group looked at their first 200 cases, using
a different technology than we have in this country. Their early
data shows local recurrence rates similar to rates with external
beam therapy. Local recurrences overwhelmingly occur near the original
cancer, so the idea of trying to not radiate the other three quadrants
of the breast makes a lot of sense.
There also have been great improvements in systemic therapy, which
affects the remainder of the breast. My early guess is that intraoperative
radiation therapy will be as effective as external beam and will
be embraced rapidly once we work out the optimal technology.
Evaluating results of the ATAC adjuvant
trial in postmenopausal women
The headline news from the ATAC trial is that anastrozole looks
better than tamoxifen, and the combination does not look any different
than tamoxifen. This confirms the findings in the stage IV setting
showing that anastrozole was at least as good as, if not better,
than tamoxifen and certainly had a more favorable side-effect profile.
We have been using aromatase inhibitors at Memorial Sloan- Kettering
since 1995 when anastrozole was approved, and these agents are very
well tolerated. They don’t cause nearly the side effects that
our patients on tamoxifen tell us about, and we’ve been enthusiastic
about using them.

Future trials of aromatase inhibitors
in DCIS
I’m confident that DCIS will be the first arena that we
move into with the aromatase inhibitors beyond invasive breast cancer.
The RTOG has a DCIS trial looking at women with small favorable
lesions randomized to radiation or not. This trial requires that
all women take tamoxifen. They couldn’t accrue enough patients,
and ultimately they had to remove the tamoxifen requirement. This
gets back to tamoxifen’s image problem. I think that the community
will embrace an aromatase inhibitor trial in DCIS. The prospect
of an agent with a better side-effect profile than tamoxifen is
very exciting in both the DCIS and the chemoprevention settings.
Select Publications
|
