| You are here: Home: BCU 8|2003: Leroy M Parker, MD
Edited comments by Dr Parker
Case discussion: 79-year-old woman with ER-positive metastatic disease following adjuvant tamoxifen
History
This patient was initially diagnosed in her late sixties with a 3-centimeter History Stage II breast cancer. She was treated with breast conservation surgery and participated in NSABP-B-18, comparing preoperative versus postoperative doxorubicin/cyclophosphamide. She received AC postoperatively and tolerated it without any major difficulties. She received five years of adjuvant tamoxifen, which she also tolerated without complaints.
Following tamoxifen the patient was disease-free for three years. She was not under the care of an oncologist, but rather a primary care physician who didn't recognize that her increasing “arthritic” symptoms were actually the slow onset of bony metastases. This went on for a year and finally the appropriate studies were performed, including a bone scan, which revealed disease throughout her skeleton, but not in any visceral organ.
She was a vigorous and independent individual at the time I treated her adjuvantly and when she presented with progressive disease. When she returned to me with metastatic disease, her independence was threatened, and she was disappointed that the recurrence had not been detected earlier. Like many patients, she thought that after five years of being disease-free, there was no need to worry about breast cancer.
Follow-up
We discussed the possibility of enrolling her in a clinical trial comparing Follow-up fulvestrant and anastrozole, but we needed to demonstrate whether her disease was sensitive or refractory to tamoxifen, so she was placed on tamoxifen and had objective progression in three months.
At that point, she entered the study and was randomized to what we later learned was fulvestrant. She began feeling better within about three months, her tumor markers — CEA and CA-27.29 — decreased from in the hundreds to the normal range; she had radiographic evidence of bone healing, and she no longer required pain medication.
She maintained this excellent response for three-and-a-half years, and then her tumor markers began to rise and she experienced increased discomfort. We unblinded the study and offered her the alternate drug, anastrozole. She once again responded to treatment, which lasted about 18 months.
This time when she progressed I chose to start her on chemotherapy rather than another hormonal agent because of the degree of her pain, and my interest in bringing the disease under control without resorting to radiation therapy. She received a series of single-agent chemotherapy regimens, including vinorelbine, which she tolerated very poorly. She experienced GI intolerance, which occurs in about 15 percent of my patients.
Discussion
This patient had a very dramatic response to fulvestrant that lasted nearly Discussion four years. I had the pleasure of participating in the double-blind, double-dummy trial in the United States comparing fulvestrant and anastrozole in patients with tamoxifen-resistant disease.
The results of the European and North American studies demonstrated that fulvestrant and anastrozole are equivalent in a controlled clinical trial, but there is a suggestion that duration of response may be somewhat longer in patients on fulvestrant. This is a tantalizing piece of data that needs to be looked at further.
It's great to have fulvestrant as another option for patients who progress following adjuvant tamoxifen, as well as for patients with whom compliance or availability of drugs is an issue. Also, in patients receiving drugs such as pamidronate or zoledronate for bone metastases, the fulvestrant injection can be administered when they are in for treatment, and we know they're receiving adequate care.
In terms of tolerability of the injections, I have observed absolutely no problems with them and have received almost no complaints from patients who are receiving the medication. Hot flashes can be difficult to control in many women who have had prior hormone replacement therapy, and I find they're equivalent whether the patient is taking anastrozole or fulvestrant in the metastatic setting. One of fulvestrant's most important qualities is that it does not have any agonist activity, so it doesn't adversely affect the endometrium.
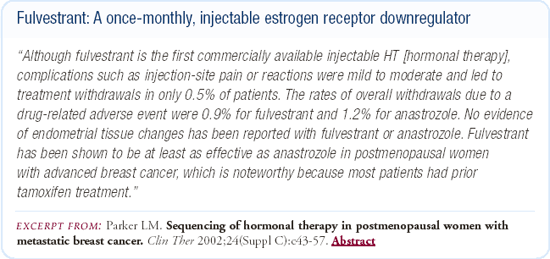
Sequencing fulvestrant in women with hormone-responsive disease
A number of studies demonstrate that patients who have benefited from fulvestrant at the time of progression can respond to any number of other hormonal agents. Conversely, patients who have not benefited from it can also respond to other hormonal agents. In addition, we have some evidence that fulvestrant is an effective drug after progression on an aromatase inhibitor.
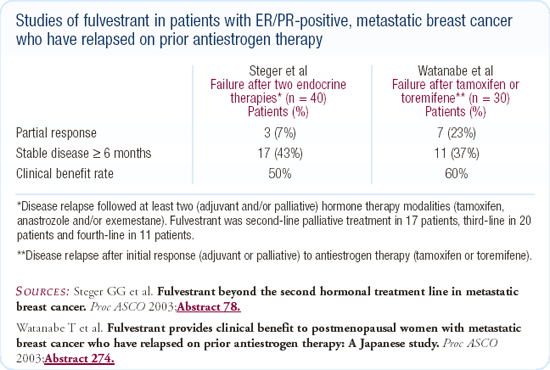

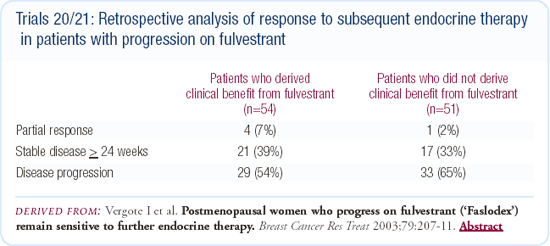
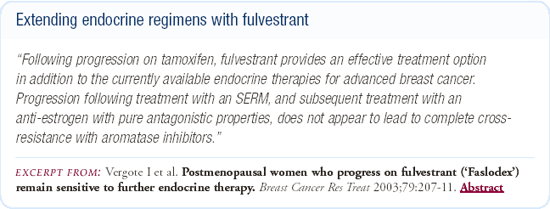
First-line trial of fulvestrant versus tamoxifen
Data have been presented demonstrating that fulvestrant is active in the first-line setting, but in the first-line study comparing it to tamoxifen, it did not prove to be more active. The primary endpoint was time to treatment failure, and tamoxifen was superior, although not statistically. One question that has been raised in this setting is whether the fulvestrant dose was adequate.
A number of investigators feel that some of the early failures seen in the comparison of fulvestrant and tamoxifen might indicate that patients were not brought up to their steady-state level, and that a loading dose of fulvestrant may be necessary.
This is currently being studied in a clinical trial that gives patients a loading dose in the first month of therapy. I would not recommend the concept of a loading dose in a nonprotocol setting at this time. We already know that when fulvestrant was compared to anastrozole as treatment for progression after tamoxifen, the current dose was adequate.
Fulvestrant in the adjuvant setting
I'm sure fulvestrant will be studied in the adjuvant setting at some point. There are a number of investigators who are quite interested in combining an aromatase inhibitor with fulvestrant to fully deplete estrogen. Despite the results of combined hormonal therapy in the ATAC trial, I'm convinced that there is a biologic basis for investigating an aromatase inhibitor and fulvestrant combination. It remains to be seen whether it's going to play out in a positive way in the metastatic setting.
CALGB-9741: Dose-dense chemotherapy
I participated in the CALGB-9741 trial and was very impressed by the results and the ease with which patients can be treated with dose-dense chemotherapy. I utilize the dose-dense approach in the nonprotocol setting, specifically the combination rather than the sequential regimen. I've been using filgrastim, but we are about to perform a study with pegfilgrastim to evaluate its safety and efficacy in a larger number of patients.
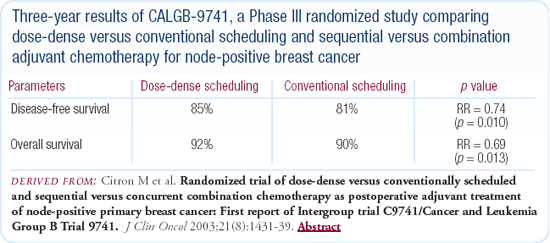
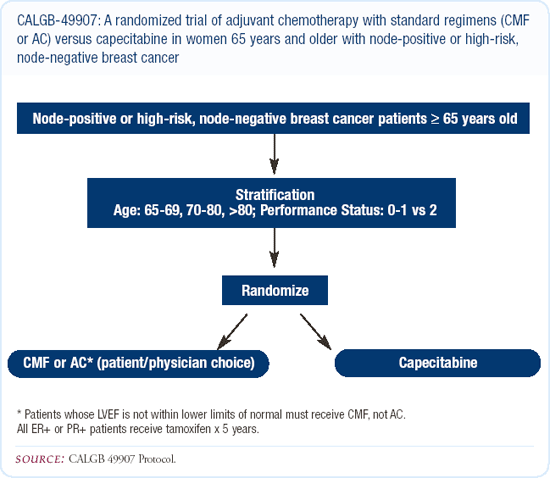
Adjuvant chemotherapy in the elderly patient
I find that if an elderly patient's performance status is good when they begin treatment, they'll do just as well as younger patients in tolerating adjuvant chemotherapy. On the other hand, if they have significant comorbidities, I am more hesitant to embark on chemotherapy, particularly in patients with estrogen receptor-positive disease. I participate in the CALGB trial randomizing elderly patients to capecitabine versus CA or CMF.
Having used capecitabine in the metastatic setting, I can attest to the fact that it's a highly effective drug, and I believe it will have an impact on early breast cancer. Patients need to be a partner when using this drug, because they have to recognize when they're beginning to experience toxicities so that we can tailor the dose accordingly.
Trastuzumab in combination with carboplatin and paclitaxel
In a patient with HER2-overexpressing, ER-negative breast cancer who is not eligible for a study, the data on the combination of carboplatin, paclitaxel and trastuzumab is very important. In these situations, the question always comes up as to whether the extra toxicity from a combination will be worth it and whether the sequential use of the drugs is just as good.
This hasn't been answered by the current trials. In a number of patients with ascites and other manifestations of breast cancer that mimic ovarian cancer, I have felt comfortable using the carboplatin/paclitaxel/trastuzumab combination. Generally, I give the paclitaxel on a weekly basis.
Trastuzumab/vinorelbine combination
At our institution, we've had tremendous experience with the combination of vinorelbine and trastuzumab and found it very useful. We are participating in a clinical trial comparing trastuzumab with either vinorelbine or a taxane, and I believe we'll find similar response rates but different side effects.
I don't know whether quality of life will be superior with any one combination, but my clinical impression is that trastuzumab/vinorelbine is extremely well-tolerated by the vast majority of patients. I believe at acceptable paclitaxel doses, trastuzumab with paclitaxel is also well-tolerated in most patients; I have observed more problems with weekly docetaxel and trastuzumab.
Management of HER2-overexpressing, ER-positive metastatic breast cancer
It's very clear to me that patients with HER2-overexpressing, ER-positive disease benefit from combining chemotherapy and trastuzumab. My first-line approach is to participate in a clinical trial, if possible, and the trial currently open at our institution is a Phase I/II study of trastuzumab plus flavopiridol, a cyclin inhibitor. At this point we don't have the data to say that we've made a lot of headway, but it's a very interesting concept.
Flavopiridol is not yet clinically available, and it's the first cyclin inhibitor to be studied in clinical trials. Some information is available on cyclin overexpression and prognosis in breast cancer. There's clear involvement of these factors in the biology of cancer, and this is our first attempt to block them. Most of the data on flavopiridol is derived from treatment of lung cancer.
Continuing trastuzumab in the metastatic setting beyond disease progression
Often I continue patients on trastuzumab beyond disease progression and switch the chemotherapy agent. I've had patients taking trastuzumab for three or four years, while switching the chemotherapy agents.
When a patient plateaus after an initial response to chemotherapy, I generally use trastuzumab alone. I continue the trastuzumab indefinitely or until progression, often using an every-three-week schedule.

Select publications
|
