| You are here: Home: BCU 8|2003: Hope S Rugo, MD
Edited comments by Dr Rugo
Gene expression profiling and treatment outcome
Dr Lajos Pusztai's data from MD Anderson indicate that in the future we will be able to look at the genetic makeup of patients with breast cancer and have a better idea of their treatment outcome. Data already indicated that prognosis could be predicted from a gene array.
Dr Pusztai found that women who had a complete pathologic response to neoadjuvant chemotherapy women who might have a better prognosis were most likely to have a specific gene array. About 75 percent of the women with the specific gene array had a pathologic complete response. In contrast, only 20 percent of the women who did not fit into that particular pattern of gene expression had a pathologic complete response.
Resistance to systemic therapy
At UCSF, an NCI-funded study will look at tumor tissue obtained from women treated with trastuzumab alone, examining the genetic makeup of these tumors to try to understand what creates trastuzumab resistance. In women with HER2-positive breast cancer, resistance may include other pathways being activated.
Research in animal models and in vitro testing suggests that the effect of epidermal growth factor receptor (EGFR) upregulation on hormone resistance is important. Some patients have primary endocrine resistance because of upregulation of EGFR.
There are studies combining hormonal agents with agents that block EGFR. A study of fulvestrant and gefitinib a very exciting combination will look at the reversal of hormone resistance. It would also be interesting to look at blocking the production of estrogen in that setting with an aromatase inhibitor.
Fulvestrant in women with metastatic breast cancer
I tend to use fulvestrant as second- or third-line therapy. Because most of our patients have oral medications covered by insurance, coming in once a month for an injection is a bigger issue. I've also been interested in the pharmacokinetics of fulvestrant. The trial evaluating it in combination with gefitinib will administer fulvestrant every two weeks for the first few doses, because the pharmacokinetics indicate that it may take up to three months for fulvestrant to reach steady state serum concentrations. We've already seen that fulvestrant is effective, but it's not better than the aromatase inhibitors. It may just be a pharmacokinetic issue; by dosing fulvestrant more frequently, we may see improved efficacy in this trial.
Fulvestrant's tolerability
When using second- and third-line agents, we often don't see many side effects. Since these patients have advanced disease, they are more worried about response. I have seen very few side effects, other than hot flashes, with fulvestrant.
We usually use two 2.5-cc injections of fulvestrant, because many nurses in the United States are not comfortable giving the whole 5-cc injection.
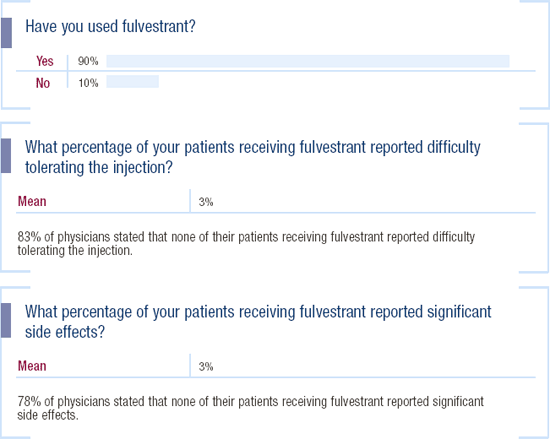
2003 Survey of US Oncologists
Use and Tolerability of Fulvestrant
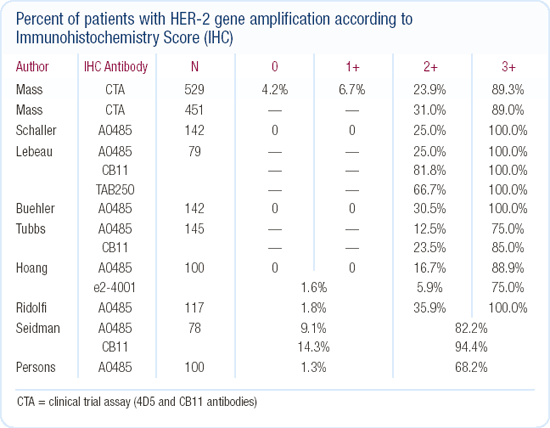
Algorithm for HER2 testing
At our institution, we perform HER2 testing by immunohistochemistry (IHC). If the patient's tumor scores 2+ on IHC, we perform a FISH test. For quality assurance purposes, some tumors scoring 1+ or 3+ are also tested by FISH, but we do not do this routinely. In our patients with tumors that score 2+, about 20 to 30 percent are truly HER2-positive. This is consistent with the results from the trastuzumab pivotal trial.
Treatment of women with ER-positive, HER2-positive metastatic disease
If a postmenopausal woman with ER-positive, HER2-positive metastatic disease presents with a minimal tumor burden, I will treat her with an aromatase inhibitor initially and wait to use trastuzumab. I usually start with a nonsteroidal aromatase inhibitor letrozole or anastrozole and then move on to exemestane or fulvestrant in patients whose disease progresses.
In patients who need chemotherapy, we use a combination of chemotherapy and trastuzumab, because the pivotal trial data demonstrated an improvement in survival for the combination. When the patients are ready to discontinue chemotherapy, we use the next sequential hormonal agent as maintenance therapy in conjunction with trastuzumab. Studies are currently evaluating the effectiveness of trastuzumab in combination with the aromatase inhibitors, and the results will be very interesting.
Trastuzumab monotherapy in patients with HER2-overexpressing metastatic breast cancer
If patients have fairly low-bulk disease bone and soft-tissue and are minimally symptomatic, we will try trastuzumab as a single agent. Trastuzumab monotherapy is a good transition therapy for patients who have just learned that they have metastatic disease.
I start with weekly trastuzumab because I want the serum concentrations to rise quickly. Thereafter, as long as they're doing fine, I switch to every-three-week trastuzumab. We have several women who have been on trastuzumab for more than two years as their only treatment for metastatic disease; this is really remarkable. We encouraged another woman, who initially received a taxane with trastuzumab, to subsequently be treated with trastuzumab alone, because she primarily had bone disease. She received chemotherapy because she was 32 years old. She's been on trastuzumab alone for three years, and in these patients, trastuzumab monotherapy is very reasonable.
I recently treated a patient with lung nodules who had a lot of disease after neoadjuvant AC and paclitaxel. I wasn't very enthusiastic about starting chemotherapy too early because I believed she had relatively resistant disease. I started her on trastuzumab alone, which has controlled her disease for about eight months. She's now moving on to chemotherapy.
Trastuzumab in combination with chemotherapy
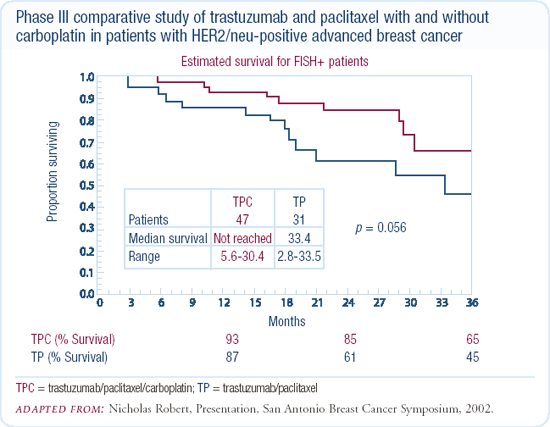
I use chemotherapy up-front in patients with life-threatening or very bulky HER2-positive disease. In these patients, chemotherapy selection depends on their adjuvant treatment. Traditionally, we'll start with a taxane and trastuzumab. For patients in visceral crisis or with bulky disease, I've been adding weekly carboplatin. Although Nick Robert's randomized trial evaluated an every-three-week schedule, we see a fair amount of thrombocytopenia with that treatment schedule, so we've been using weekly carboplatin, paclitaxel or docetaxel, and trastuzumab. Once the patients have a good response, we discontinue the chemotherapy and continue with every-three-week trastuzumab alone.
I also use capecitabine with trastuzumab, and it's been very effective. Patients with HER2-overexpressing disease are often very receptive to capecitabine. So it's important to use that drug as part of the treatment approach for these patients.

Management of patients who progress on trastuzumab and chemotherapy
There are a whole host of drugs to try in women progressing on trastuzumab and chemotherapy. We certainly use vinorelbine and gemcitabine with trastuzumab. I've given one patient, who had particularly resistant disease, the combination of gemcitabine and a taxane with trastuzumab. We're more comfortable using carboplatin early in the course of therapy. For patients who haven't been treated previously with an anthracycline, I'll stop the trastuzumab and use either weekly epirubicin or liposomal doxorubicin.
My colleagues in the community frequently ask whether to continue the trastuzumab as these patients progress, and we don't know the answer. The study presented by Debu Tripathy several years ago in San Antonio, of patients who continued trastuzumab, really didn't provide us with any definitive information. Interestingly, Dr Pusztai tried to conduct a multicenter trial in patients who progressed on a taxane and trastuzumab regimen. The trial design was to randomize patients to vinorelbine or vinorelbine with trastuzumab, but he couldn't enroll patients because they didn't want to discontinue trastuzumab.
Some patients may still benefit from trastuzumab beyond progression. There have been some anecdotal reports of radiation sensitivity and slowing of disease with trastuzumab, so that when trastuzumab is discontinued, the disease seems to grow faster. Although this is all anecdotal, it makes patients reticent to stop trastuzumab.
2003 Survey of US Oncologists
Continuation of Trastuzumab upon Disease Progression
Central nervous system metastases
Brain metastases are a big issue in the control of breast cancer, and we really need drugs that can cross the blood-brain barrier. Novel taxanes are being studied, a variety of which cross the blood-brain barrier. Those are very exciting, because they can be used in the adjuvant setting as prophylaxis. Other drugs get into the cerebral spinal fluid (CSF), such as capecitabine. One of the problems with capecitabine is that we don't know how much of it goes into the CSF because it's a prodrug. The other drug I've been interested in is irinotecan. It's been tested in combination with capecitabine for primary brain tumors, and it clearly goes into the CSF.
Sequential single-agent versus combination chemotherapy
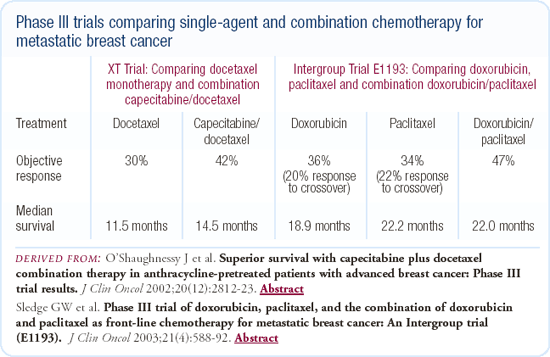
Combination chemotherapy is important for patients in visceral crisis who need a rapid response. Combination chemotherapy improves response, and it is generally more toxic. It does not improve quality of life sufficiently to make up for the fact that it doesn't prolong survival. The capecitabine/ docetaxel study published by Joyce O'Shaughnessy is the only study that has demonstrated some prolongation in survival. That trial was problematic because of the absence of sequential therapy in the women randomized to the docetaxel-alone arm. It may be that the survival benefit wouldn't have occurred if the patients on the docetaxel-alone arm had capecitabine available.

Select publications
|
