| You are here: Home: BCU Surgeons Vol.2 Issue 4: Aman Buzdar, MD, FACP
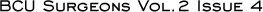
Edited comments by Dr Buzdar
Updated data from the ATAC trial: 47-month follow-up
The initial publication of the ATAC results caused concern because the data represented only about two-and-a-half years of follow-up. Now the median follow-up is four years, there are no new safety concerns and the early efficacy advantages have persisted - in fact, the absolute differences are increasing with time. I believe the data provide strong support for the adjuvant use of anastrozole in postmenopausal patients with hormone receptor-positive, early-stage breast cancer.
The divergence of the curves is not unique to the ATAC trial. The Oxford overview of chemotherapy data or ovarian ablation demonstrates a persistent divergence of the curves with time. I believe this is what happens with a successful adjuvant therapy. Basically, there is a reduction in events and, in a fraction of patients, micrometastases are eliminated by the systemic therapy. It takes time for the patients receiving the alternate therapy to show evidence of recurring disease, and when that happens, you see this pattern of divergence.
Recurrence and mortality data from the ATAC trial
Patients on anastrozole had fewer local and distant recurrences and fewer second primary breast cancers, but more follow-up is required. When there are more than 704 distant recurrences between the two arms, we should be able to determine the effect of the therapies on systemic recurrences. The numeric differences already favor anastrozole, both with an intent-to-treat analysis and when looking at ER-positive patients, and I expect that overall survival will parallel the disease-free survival pattern. Currently, there are very few deaths, but when there are more than 704 deaths between the two monotherapy arms, the survival data will also be unblinded.
Implications of the ATAC trial in clinical practice
As an academic and a practicing clinician, my role is to be candid with my patients and let them be an active participant in their treatment decisions. Since the initial results of the ATAC trial were reported, I have discussed them with my patients, including the benefits of anastrozole and the effects on bone. In my practice, about nine out of 10 women choose anastrozole over tamoxifen.
I believe the ASCO Technology Assessment is very conservative, and I respectfully disagree with their recommendations. The data favoring anastrozole is very strong and is already impacting clinical practice and research. There is a very large Canadian trial taking place in which the control arm is anastrozole rather than tamoxifen.
Use of other aromatase inhibitors in the adjuvant setting
Currently, I do not recommend the use of aromatase inhibitors other than anastrozole in the adjuvant setting. I recently published a review in Cancer demonstrating differences in the pharmacology and pharmacokinetics among the newer generation of aromatase inhibitors - anastrozole, letrozole and exemestane. Until we have long-term safety and efficacy data on letrozole and exemestane, I don't recommend their use outside of a clinical trial.
Experimental data in mice show possible benefits of exemestane on the bone, but this still needs to be proven in patients. In addition, exemestane is a steroidal molecule that, because of its agonistic effect, may have safety issues similar to tamoxifen. We don't have enough in long-term safety or efficacy data, even in metastatic disease, to know whether these androgenic effects will be beneficial or detrimental when exemstane is given to patients for a long period of time.
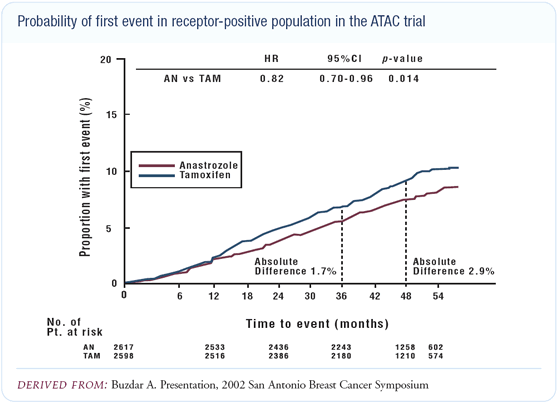
Use of bisphosphonates in patients on estrogen deprivation therapy
An Austrian group evaluated the effect of bisphosphonates in premenopausal patients who received LHRH agonists and tamoxifen or anastrozole. Significant prevention of the bone loss and bone-related events was seen in the patients who received the bisphosphonate.
For patients on anastrozole, the key is to evaluate baseline bone density and then follow these patients. If and when there is a change, effective therapies can be implemented to prevent further bone loss. A number of effective bisphosphonates are available. The EORTC is comparing exemestane to tamoxifen, and we are participating in the bone subprotocol. In this study, patients have a bone density evaluation up front and at regular intervals to monitor changes in bone.
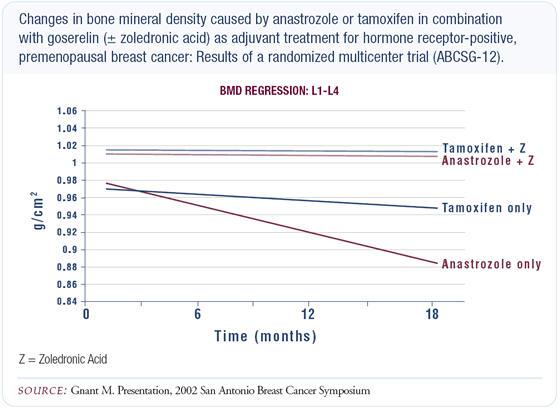
Adjuvant endocrine therapy in premenopausal women
The majority of premenopausal patients go into premature ovarian failure after chemotherapy; however, in a small fraction of those who are young, ovarian function remains intact. At this time, the evidence is very weak for utilizing LHRH agonists with tamoxifen in that setting; however, there are several large, multinational studies taking place that will provide that answer in the next few years. Even if a premenopausal patient becomes amenorrheic, one should not assume she is postmenopausal, because patients can experience transient amenorrhea. LH, FSH and estradiol levels should be measured and unless they all fall in the postmenopausal range, it is not appropriate to use an aromatase inhibitor. Women who become amenorrheic but maintain borderline levels may still regain ovarian function.
An ongoing trial is examining whether the aromatase inhibitors (with ovarian ablation or suppression) will have a favorable impact on the disease-free and overall survival in hormone-receptive, premenopausal patients. Until we have that answer, we will all make different treatment decisions in an effort to select the best therapy for our patients without definitive data to guide us. My general, nonprotocol approach to an ER-positive, premenopausal woman with multiple positive nodes is to offer tamoxifen in addition to chemotherapy.
Select publications
|
