| You are here: Home: BCU Surgeons Vol.2 Issue 4: Editor's note
 |
Editor’s Note |
|
| Half empty or half full? |
We have to wait for the data from our NSABP-B-17 DCIS protocol to mature to determine if radiation therapy decreases the incidence of invasive recurrence. Meanwhile, our new DCIS protocol, B-24, just started a week ago and although we don't have the results from B-17, we had to select a control arm for B-24, which will be lumpectomy plus radiation. The B-24 trial will compare tamoxifen to placebo. The study is provocative, but I will pursue it with enthusiasm because I think, biologically, it is very interesting and will give us a great deal of very needed information. DCIS is being detected much more frequently, but we really don't know how to treat the disease. Our work is cut out for us, and we all look forward to the day when advances in molecular genetics and in the biochemistry of cancer will make today's discussion seem like a very primitive exercise.
- Interview with Norman Wolmark, MD
Breast Cancer Update, May 1991
During my monotonous drive up the Florida Turnpike from Miami to Orlando on the way to the June 2003 NSABP Annual Group Meeting, I decided to dust off and listen to an interview I conducted with Dr Norm Wolmark more than a decade ago. At that time, the NSABP had just launched three potentially paradigm-shifting trials: the high-dose adjuvant chemotherapy B-22 study, the preoperative chemotherapy B-18 trial and P-1, the "tamoxifen prevention study." By now, we all know the results of these studies. Like other high-dose chemo attempts, B-22 was a total bust; B-18 left perhaps a promissory note for the future and P-1 delivered. One out of three ain't bad when you're trying to shift paradigms, and I considered how other recent research advances would fit into the historical perspective of breast cancer management.
In this program, Dr David Krag reviews the NSABP sentinel node B-32 trial. While this procedure has greatly benefited patients by reducing operative morbidity, it is unlikely to impact mortality. Dr Krag also reviews his laboratory research on targeted cancer strategies. This discussion - like so many I hear from contemporary breast cancer research leaders - is fascinating biology, but one wonders when Dr Wolmark's 1991 wish will be fulfilled, and we will begin to see these approaches translated into patient-care advances.
Dr Wolmark's above comments on DCIS are also interesting in a historical perspective. B-17 eventually did demonstrate an advantage to radiation therapy, but skeptics such as previous Breast Cancer Update interviewee, Dr Mel Silverstein, still believe that many patients can be managed with lumpectomy alone. NSABP-B-24 demonstrated that tamoxifen reduced local recurrence and contralateral disease.
Our continuing medical education group is also interested in the issue of how clinical breast cancer research is translated into practice, and in that regard, for the second year, we conducted a national patterns of care telephone survey in July of this year. One hundred surgeons from our mail list were randomly surveyed on a variety of management issues, and the data are presented in this booklet starting on page 22.
The results of this survey provide a fascinating glimpse into current management patterns. With regard to DCIS, most patients are receiving tamoxifen, and Dr Wolmark discussed NSABP-B-35 that is comparing this agent to anastrozole in an attempt to verify that the advantages of this aromatase inhibitor demonstrated in invasive disease are also seen in noninvasive breast cancer. Our patterns of care survey demonstrates optimism that an advantage will be observed (see page 23).
The survey also reveals that sentinel node biopsy is now firmly entrenched in community care (see page 30) and that there is considerable heterogeneity in how surgeons approach clinical practice. Our group will utilize these findings as part of our needs assessment for upcoming education programs.
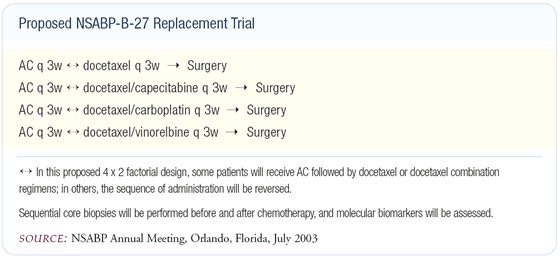
The NSABP meeting included what many will consider a bold step in breast cancer clinical research: a proposed preoperative trial that will focus on intratumoral markers of response in an attempt to accelerate the timetable for identifying effective adjuvant or neoadjuvant therapies (see figure below). At this same meeting, the group discussed plans for a new colorectal adjuvant trial that will include the anti-VEGF agent, bevacizumab, and Dr Wolmark told me that a similar trial may be considered in breast cancer in the future.
Is this news from the "frontline of the war on breast cancer" encouraging or discouraging? I would be interested in your point of view.
-Neil Love, MD
(NLove@ResearchtoPractice.net)
|
