| You are here: Home: BCU 1|2004: Aron Goldhirsch, MD

Edited comments by Dr Goldhirsch
International Breast Cancer Study Group (IBCSG) trials
Because we believe in tailored treatment, our group conducts trials within biologically homogeneous populations. Currently, we have three sets of trials: (1) studies for patients with endocrine-responsive disease, (2) trials for patients with endocrine-unresponsive disease and (3) protocols for patients with local recurrences.
Postmenopausal women with endocrine-responsive breast cancer
We completed accrual to an adjuvant trial (IBCSG-18-98) comparing five years of tamoxifen, five years of letrozole, two years of tamoxifen followed by three years of letrozole, and two years of letrozole followed by three years of tamoxifen in postmenopausal patients with endocrine-responsive disease. This trial accrued 8,028 patients (Slide 13, page 37).
A lifelong treatment strategy for patients with an increased risk of breast cancer recurrence might be reasonable. I think maintaining the cells under control and suppressing new tumors requires a sequential approach that includes endocrine therapy for tumors that are endocrine responsive.
Premenopausal women with endocrine-responsive breast cancer
In premenopausal women with endocrine-responsive disease, we initiated three adjuvant trials in August 2003. The Suppression of Ovarian Function Trial (SOFT), the Tamoxifen and Exemestane Trial (TEXT) and the Premenopausal Endocrine Re- sponsive Chemotherapy Trial (PERCHE).
The SOFT trial will compare tamoxifen alone to ovarian function suppression plus tamoxifen and ovarian function suppression plus exemestane. This trial was designed specifically for oncologists who view tamoxifen as standard therapy (Slide 4, page 33).
The TEXT trial will compare ovarian function suppression plus tamoxifen to ovarian function suppression plus exemestane. These patients may or may not receive chemotherapy (Slide 5, page 33).
The PERCHE trial will determine whether adjuvant chemotherapy is necessary. Premenopausal women are randomly assigned to chemotherapy or no chemotherapy (Slide 6, page 34). Adjuvant chemotherapy selection is left entirely up to the investigator, and endocrine therapy consists of ovarian function suppression with tamoxifen or exemestane. Patients may also be randomized to TEXT for endocrine therapy.
Women with a resected locoregional breast cancer recurrence
The Swiss trial comparing adjuvant tamoxifen to observation in patients with endocrine-responsive local recurrences demonstrated an advantage for the patients receiving adjuvant tamoxifen compared to those treated with only local therapy. We are conducting a trial with NSABP to evaluate the benefit of adjuvant chemotherapy in the treatment of patients with local recurrences (Slide 10, page 36).
Patients are randomly assigned to chemotherapy or observation after completing local treatment for the recurrence. Several chemotherapy regimens can be used for a duration of six months. Patients with endocrine-responsive disease will also receive adjuvant endocrine therapy. I think chemotherapy will provide an advantage for patients with endocrine-unresponsive disease.
Women with endocrine-unresponsive disease
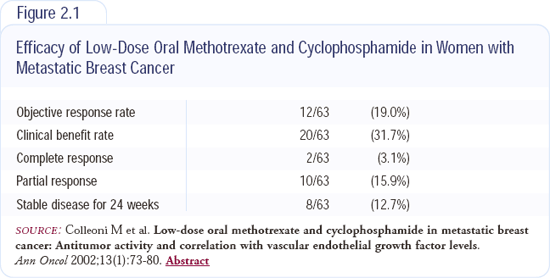
In patients with endocrine-unresponsive disease that has HER2-overexpression, the HERA trial will compare one or two years of adjuvant trastuzumab to observation after the completion of adjuvant chemotherapy (Slide 7, page 34; slide 8, page 35). In patients with endocrine-unresponsive and HER2-negative disease, another trial will randomly assign patients after completion of adjuvant chemotherapy to observation or one year of low-dose metronomic cyclophosphamide and methotrexate (Slide 9, page 35). This inexpensive regimen has been proven in two series of patients with advanced disease (Figure 2.1).
Metronomic chemotherapy
Metronomic chemotherapy has some antiangiogenic effects. Bob Kerbel will soon publish new information on the induction of antiangiogenic effects by low-dose chemotherapy. In the Annals of Oncology (January 2002), we published the results of a trial with about 60 patients treated with low-dose oral cyclophosphamide and methotrexate. In pretreated patients, the clinical benefit rate was about 30 percent. Two patients with biopsy-proven liver metastases had a complete remission. The patients experienced occasional Grade I leukopenia.
Globalization of breast cancer clinical research
The SOFT, TEXT and PERCHE trials have led to a unification of research efforts. This is the first time that globalization has been recognized as a priority for everyone, and everyone made the jump. This has been quite impressive.
Pregnancy in women who have been diagnosed with breast cancer
According to case reports and cohort studies, pregnancy after a diagnosis of breast cancer has been associated with an improved prognosis. Obviously, prospective studies have not been conducted and could only be accomplished through a registry of all pregnancies. The concern about pregnancy leading to a breast cancer recurrence is not substantiated by any data.
Case comment: Nonprotocol management of a 32-year-old woman with resected ER-positive, node-positive breast cancer
A 32-year-old woman has a long life expectancy, and I would recommend a full course of chemotherapy and endocrine therapy for such a patient. I would probably use an LHRH analog plus tamoxifen. In advanced disease, data show an advantage for an LHRH analog plus tamoxifen compared to tamoxifen alone. In the adjuvant setting, Nancy Davidson's data are compelling, although her trial did not have a tamoxifen-alone arm. Additionally, the Austrian trial demonstrated that an LHRH analog plus tamoxifen was much better than chemotherapy.
In a premenopausal woman with low-risk, endocrine-responsive disease, I would consider adjuvant ovarian ablation or suppression plus tamoxifen without chemotherapy. Endocrine-responsive disease is less likely to be of high metastatic potential; therefore, I would prefer endocrine treatment. Thirteen studies have shown this approach to be completely legitimate.
Predicting response to chemotherapy
For a long time it has been speculated that an increased labeling index predicts for response. Mark Lippman was the first person to receive credit for that concept. Data reported in Milan indicate that high proliferation rate, as expressed by Ki-67, predicts for response to chemotherapy. Endocrine unresponsiveness also predicts for response to chemotherapy. For example, women with endocrine-unresponsive disease have a complete remission rate that is four or five times greater than those with endocrine-responsive disease. Attempts are also being made to determine a gene profile that will predict for response to docetaxel. This should be done, but we are still in the very early stages.
Neoadjuvant therapy
We are evaluating chemotherapy, chemotherapy plus endocrine therapy, and endocrine therapy alone in both premenopausal and postmenopausal women. Our pathology group is conducting a very thorough biological dissection of all features, preoperatively and postoperatively.
We use neoadjuvant endocrine therapy only in a protocol setting, because we are unsure of the duration of exposure required to obtain the maximum effect. With chemotherapy, a response is evident within three months; this is not the case with endocrine treatment. Neoadjuvant endocrine therapy is trickier in terms of obtaining tumor shrinkage in order to obtain breast conservation.
Concomitant versus sequential hormonal therapy and chemotherapy
The most important clinical trial results reported last year were from SWOG-8814, which evaluated the concomitant or sequential use of chemotherapy and tamoxifen. That trial proves that even with a doxorubicin-containing combination, tamoxifen should be administered sequentially. Each therapy reduces the effect of the other. In the retrospective analyses of the trials in which tamoxifen and cytotoxics were combined, the effect was devastating.
Select publications
|
| Dr Goldhirsch is a Professor at the University of Bern in Switzerland; Chairman of the Scientific Committee of the International Breast Cancer Study Group; Director of the Department of Medicine at the European Institute of Oncology in Milan, Italy; and Head of the Division of Medical Oncology at the Oncology Institute of Southern Switzerland in Lugano, Switzerland. |
|
