| You are here: Home: BCU 1|2004: Denise A Yardley, MD

Edited comments by Dr Yardley
Replacing anthracyclines with taxanes in the adjuvant setting
One of the most interesting questions in adjuvant therapy is:
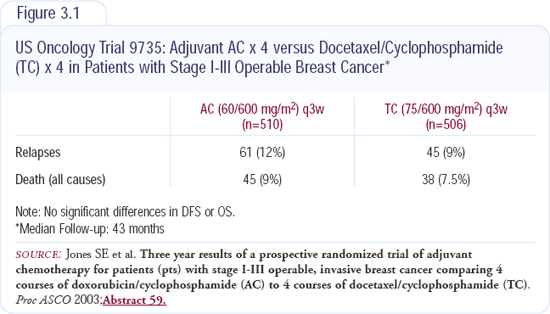
Can a taxane replace an anthracycline? The US Oncology trial presented by Stephen Jones evaluated docetaxel and cyclo-phosphamide versus doxorubicin/cyclophosphamide. In a population of patients, irrespective of HER2 status, this trial suggests that you don't need anthracyclines (Figure 3.1). The taxanes may really be usurping the role of the anthracyclines.
The BCIRG adjuvant trastuzumab trial also has a novel, nonanthracycline arm - docetaxel/platinum/trastuzumab - in an HER2-positive population. This combination is based on the preclinical in vitro data of synergism with these agents.
Adjuvant chemotherapy in a nonprotocol setting
I do not use four cycles of AC. I will consider CMF in some patients with node-negative disease who are at low risk. I usually use six-cycle anthracycline-based regimens - typically FEC. I'm looking forward to the Canadian MA21 trial data directly comparing a six-cycle anthracycline-based regimen to AC followed by paclitaxel. I think this is the "million-dollar question."
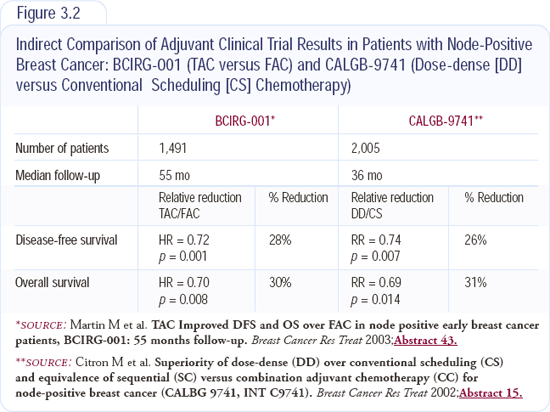
Taxanes clearly have benefit in the adjuvant setting, and I typically utilize the six-cycle TAC regimen. The disease-free and overall survival of dose-dense therapy and TAC are equivalent. Growth factor support, used in conjunction with TAC, reduces the rate of febrile neutropenia to that seen in CALGB-9741 (Figure 3.2).
Hormonal therapy in postmenopausal women
Counseling postmenopausal patients about adjuvant hormonal therapy requires a lengthy discussion. I refer to studies in the metastatic setting demonstrating a benefit to the aromatase inhibitors over tamoxifen on several endpoints, and I review the ATAC trial results and discuss the risks and benefits of the therapies and the limitations of the data.
Bone density is a big issue for patients. We aim to cure them of their breast cancer but don't want to leave them with a second problem. I monitor bone density very closely in patients on aromatase inhibitors. I also counsel patients about the side effects of tamoxifen, including endometrial cancer and thromboembolic events, especially those with comorbid conditions and a propensity for clotting.
Over the last six months, I estimate 30 to 40 percent of my patients have chosen tamoxifen and 60 to 70 percent chose an aromatase inhibitor. I believe letrozole and anastrozole are probably equivalent, but I typically use anastrozole because the ATAC data is with anastrozole.
With the recent data on tamoxifen followed by the aromatase inhibitors, this discussion is even more complicated. Some patients have misconceptions about switching after two or three years of tamoxifen. Others are relieved to know some data support changing drugs at the end of five years to give them a little bit more protection.
Chemotherapy in elderly women
We have a trial in the elderly looking at intravenous CMF every three weeks versus single-agent docetaxel. The rationale for this was an Australian trial in the metastatic setting of CMF versus paclitaxel, in which paclitaxel was the "winner." Another elderly trial in the metastatic setting demonstrated weekly docetaxel was very well-tolerated, with less than a one percent rate of febrile neutropenia.
We've had some trouble accruing to this trial, largely because the elderly patient population has so many options. They are typically hormone receptor-positive and often have indolent disease. In light of the slow accrual, we are considering closing it and letting the Intergroup trial address the role of chemotherapy in the elderly.
In the adjuvant setting, the Intergroup trial evaluating AC or CMF versus capecitabine is trying to find a more user-friendly regimen for elderly patients. The Intergroup has now built in somewhat closer patient monitoring. In looking at the elderly population, I think capecitabine is a great drug in the metastatic setting, but I believe the doses have to be modified from what is indicated in the standard package insert.
Combination versus single agent-chemotherapy in the metastatic setting
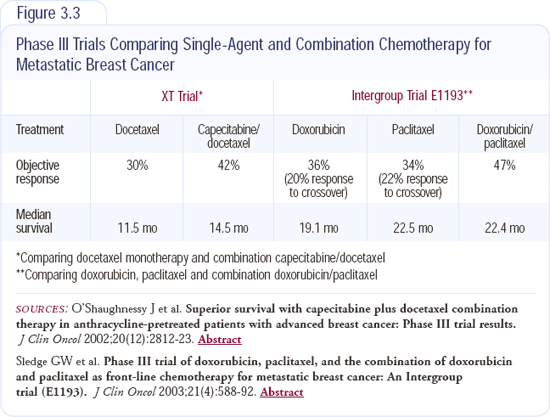
This is a big debate in oncology right now. I use combinations in some patients and single agents in others, and I believe the heterogeneity of the disease warrants that. Dr Sledge's trial demonstrated the response rate and the time to progression were significantly in favor of the combination regimen, but overall survival was equal to that of single agents with the crossover (Figure 3.3).
I may consider using a combination regimen to control the disease more quickly in very young patients, those with a very short disease-free interval, visceral disease or a large tumor burden. In the chemotherapy-naïve patient, I typically incorporate a taxane up front either as a single agent or in combination - often with a platinum.
I don't typically combine taxanes with an anthracycline up front. We have a trial of gemcitabine/carboplatin in patients previously treated with a taxane and an anthracycline, trying to use a nontaxane, nonanthracycline regimen in the first-line metastatic setting.
Sequencing of single agents in the metastatic setting is basically a patient-physician decision. I evaluate prior adjuvant therapy, the disease location and the patient's last regimen. We have data that vinorelbine following a taxane sometimes enhances peripheral neuropathy, so if a taxane was the last sequential single agent they received, I may look at capecitabine, gemcitabine or another agent.
Quality of life issues are also important in choosing the right therapy. Does the patient want to come in for a weekly therapy or might she be a better candidate for capecitabine? For example, someone trying to minimize time away from work may be a good candidate for an oral therapy. I also look at side-effect profiles. For example in a diabetic patient, neuropathy or extra steroid use may come into play.
I don't believe we have data suggesting a certain sequence to which one should adhere. The drug that's given earliest tends to have the highest response rate, and it drops sequentially thereafter.
Clinical research in targeted therapy
Our center has a very active Phase I program directed by Skip Burris. This is one of the most exciting aspects of practice, because we have very early hands-on experience with many of the novel agents.
An example of one of these agents is a very user-friendly oral drug with minimal side effects. It is a dual tyrosine kinase inhibitor, which blocks the epidermal growth factor receptors I and II. We saw durable responses in a Phase I trial in patients with trastuzumab-refractory malignancy (Figure 3.4). It has now moved into a Phase II trial in metastatic breast cancer with paclitaxel, based on data like that of trastuzumab, indicating that some targeted agents have higher response rates in combination with chemotherapy. We will also be moving into a Phase III trial in breast cancer patients.
Phase I trials are typically not designed to evaluate response, but to identify the maximum tolerated dose. With some of these targeted agents, you can deliver high doses without encountering toxicity. With agents like capecitabine and docetaxel, we realized we can dose-reduce and maintain the same outcomes. We've done skin biopsies and are evaluating biologic endpoints such as the maximum inhibition of tyrosine kinase and phosphorylation to help determine the dose to use in our subsequent trial.

Case discussion: Novel agents in a heavily pretreated patient
I am taking care of a woman in her thirties who was diagnosed with breast cancer when she was postpartum. She underwent a mastectomy, and her disease recurred in the skin. She also had some clinically positive supraclavicular nodes and a small lung nodule and liver lesion.
Her tumor expressed EGFR type I. When I began seeing her, fatigue was her biggest complaint, but she had no symptoms from the visceral involvement.
Her disease had been heavily pretreated - she had received a prior anthracycline and taxane, epothilone, gemcitabine and capecitabine. She wanted to be treated with a novel agent, and we put her on our trial of the oral dual tyrosine kinase inhibitor.
Her disease initially responded to this agent, and she maintained stable disease for almost a year. Her last scan demonstrated progression in one of the visceral lesions, so we're looking at some other options for her.
I believe she has been able to maintain an upbeat attitude because she has a life outside the hospital as a wife and a mother. It's always discouraging to tell a patient that it's time to think about another treatment because even in this time of novel agents, there are limitations in the drugs available. It is fortunate that there are so many new drugs being investigated and coming to the market, but oncology is a very humbling experience when we realize how little control we often have.
Trastuzumab with or without chemotherapy in the metastatic setting
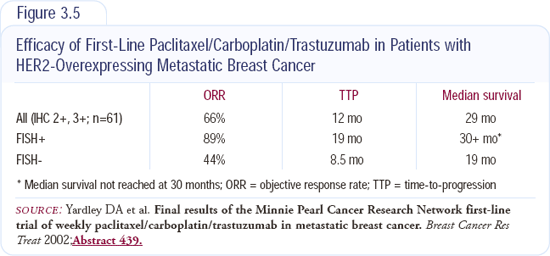
I use first-line trastuzumab with chemotherapy in patients with HER2-positive disease. Depending on the adjuvant therapy they received and the time since their treatment, I usually use paclitaxel/carboplatin with trastuzumab. We conducted a trial of this regimen on a weekly schedule (Figure 3.5), and it was well-tolerated. Nick Robert's data used an every-three-week schedule, and patients on that study had much more myelosuppression.
In the patient whose disease responds to chemotherapy and trastuzumab, I typically discontinue the chemotherapy when the patient is stable and continue single-agent trastuzumab. You can run into issues regarding how much chemotherapy to give to patients who continue to respond. I typically average about six cycles and then maintenance if there is evidence of response. When they move to trastuzumab alone, I utilize the every-three-week regimen.
Upon progression, I typically change the chemotherapy regimen and continue the trastuzumab, but we don't have randomized trial data to follow. MD Anderson tried to evaluate this with vinorelbine versus vinorelbine/trastuzumab following a taxane, but the trial closed due to poor accrual because patients didn't want to stop the trastuzumab. The little data that's out there suggest a rationale and benefit for continuing the trastuzumab and changing the chemotherapy.
HER2-positive locally advanced disease
I have not been utilizing trastuzumab up front in patients with locally advanced disease. We are conducting a neoadjuvant trial, and I believe it is an interesting concept. In evaluating the Phase II trials comparing neoadjuvant vinorelbine to vinorelbine/trastuzumab or docetaxel alone to docetaxel/trastuzumab, the pathologic response rates haven't been overwhelmingly different. Trastuzumab is not a high response rate drug, so I'm not sure it's going to be a "big home run" in changing pathologic complete response rates. I'm still awaiting the adjuvant study results.
Hormone therapy versus trastuzumab in ER-positive, HER2-positive disease
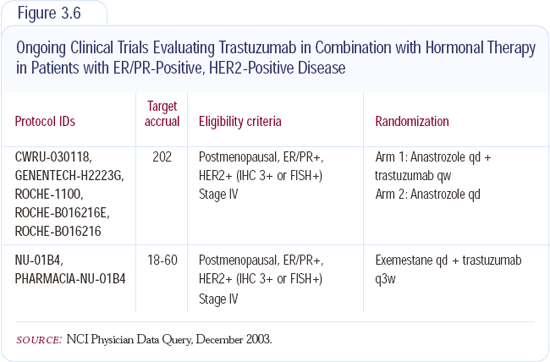
The preclinical data combining trastuzumab with hormonal agents is very exciting; however, the hormonal agents alone offer patients good quality of life in terms of being able to take a pill and not being tethered to the doctor's office. I discuss the options with my patients, and more often than not, I give hormonal therapy without encumbering them with IV trastuzumab. I will do this until the clinical trials (Figure 3.6) show that the synergism exists in the clinical setting with the combination of hormones and trastuzumab.
In a woman with rapidly progressing ER-positive disease, I tend to use chemotherapy up front, followed by maintenance with single-agent trastuzumab. Following chemotherapy/trastuzumab I may consider adding hormonal therapy to the trastuzumab maintenance. In this setting, looking at the preclinical data, I lean towards the aromatase inhbitors, although there really hasn't been a definitive trial.
Activity, tolerability and sequencing of fulvestrant
My patients like fulvestrant because it lets them get on with their activities and maintain their quality of life. In my experience, it has been much more likely to result in stable disease rather than produce measurable responses or complete remissions. However, it has stabilized patients with excellent quality of life for long periods of time without having to change therapy.
It'll be interesting to see the trials that move fulvestrant into the front-line setting. All of the hormonal agents, when they first become available, are used in patients with refractory disease.
Select publications
|
| Dr Yardley is the Director of Breast Cancer Research at the Sarah Cannon Cancer Center in Nashville, Tennessee. |
|
