| You are here: Home: BCU Surgeons 2004 Vol 3 Issue 3: Richard G Margolese, MD
Richard G Margolese, MD |
EDITED COMMENTS |
 Surgery for DCIS Surgery for DCIS
In managing DCIS, I think the most common question is: Who needs a mastectomy after breast-conserving surgery without clear margins? In my opinion, too many mastectomies are performed. In extensive cases of DCIS, mastectomies are necessary, but you can actually remove two-thirds of the breast with excellent cosmesis as long as the tumor is in the upper part of the breast. We still try to conserve a lot of the breast in patients with extensive tumors.
Role of sentinel lymph node biopsy in DCIS
Performing sentinel lymph node biopsy in patients with DCIS does not make sense. The way DCIS is currently processed, pathologically, is much improved compared to previous methods. The pathologists are performing step sections at small intervals, and it’s unlikely that we’ll miss invasive cancer. We know there’s a one percent mortality rate from DCIS, even though the most current statistics we have are from around 1990. The incidence of detecting a positive sentinel node is approximately 10 percent, but a lot of that is by immunohistochemistry. We’re not sure what that means. If you find cancer cells in the lymph nodes, you can’t dismiss it, but I’m not sure it’s going to make a big difference in DCIS.
When a mastectomy is performed for DCIS, one to three lymph nodes will likely be present in the specimen — unless you assiduously avoid those lymph nodes in the tail of the breast. Is there a concern that these are not from the sentinel node? Is it necessary to inject a tracer to make certain you have the sentinel node? I don’t think that’s a very important question these days.
DCIS and radiation therapy
Selecting patients with DCIS who require radiation therapy seems to be a debate that’s out of proportion to the problem. If you examine the retrospective studies all the way back to Lagios and Silverstein, you can identify patients who have tumors with such favorable prognostic features that they don’t need radiation. We see them in invasive cancer and 100 percent in tubular cancers. They're probably not going to need radiation therapy.
In the NSABP prospective study, every group benefited to some extent from radiation therapy, but patients with the smallest tumors and the best nuclear grade had only a small benefit from radiation therapy. The annual hazard rate went from approximately 1.85 down to 1.1, which is not large, but it is a difference. If you think radiation therapy is problematic or toxic, maybe you would withhold it from such patients. If you think it's not such a problem, you would probably give it to everybody.
Comparison of the side-effect profiles of anastrozole and tamoxifen
Anastrozole seems to be a well-tolerated and safe drug. It certainly does not carry the risk of uterine cancer, and thromboembolic events occur less than with tamoxifen. Most women receiving anastrozole do not report any problems, although hot flashes are frequently mentioned. I haven't heard complaints about arthralgias.
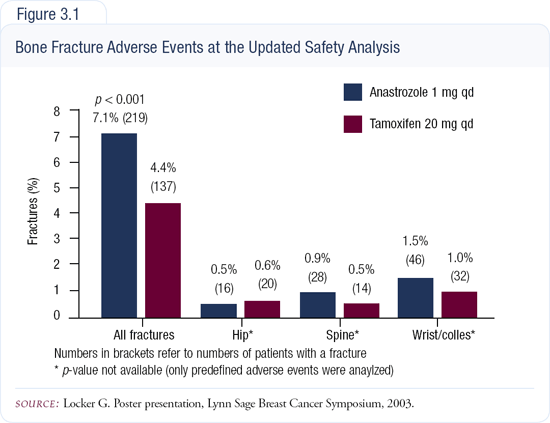
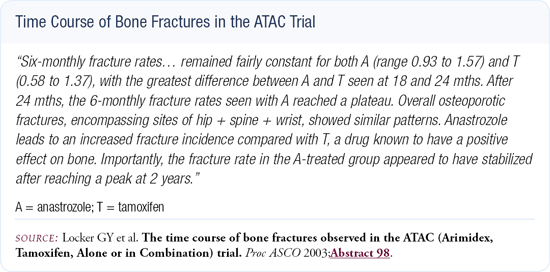
In terms of bone mineral density and the potential threat of fractures, tamoxifen results in a significant reduction in hip and wrist fractures and a slight reduction in compression fractures of the spine (Figure 3.1). The aromatase inhibitors will produce more fractures, but we don't know if this is a serious problem or not. We don't know the long-term effects of estrogen deprivation, and we don't have long-term data for patients on aromatase inhibitors. It will be important for the ATAC investigators to gather long-term toxicity data.
NSABP-B-35: Anastrozole versus tamoxifen in postmenopausal women with DCIS
NSABP-B-35 was designed shortly before the ATAC study was publicized, so data from ATAC and MA17 were not available to us. It was initiated because of the growing body of evidence that aromatase inhibitors appear to be effective in settings where tamoxifen is efficacious. Indeed, two large studies in advanced disease showed drugs like anastrozole were either equivalent to or even slightly better than tamoxifen. While we didn't have the ATAC data at the time, the dramatic reduction in second or contralateral breast cancers in women who received anastrozole versus tamoxifen is very exciting and emphasizes the importance of our trial.
NSABP-B-24, which showed a reduction in invasive cancer in the patients with DCIS who were randomly assigned to adjuvant tamoxifen versus placebo, also played a role in the development of B-35. Reducing the risk of invasive cancer is key, and while a recurrence of DCIS is unfortunate and perturbing, it is not life-threatening. We found that tamoxifen reduced this risk to just under two percent, and we had to decide just how much room there was for improvement and whether B-35 was worthwhile. It should be noted that two percent was for the entire group and to determine if women with a higher risk for invasive recurrence have a greater absolute benefit, we would need to study them separately.
Select publications
 |
Dr Margolese is Director of the Department of Oncology at the Jewish General Hospital at McGill University, Herbert Black Chair in Surgical Oncology at McGill University in Montreal, Quebec and an Executive Committee Member of the National Surgical Adjuvant Breast and Bowel Project in Pittsburgh, Pennsylvania. |
|
