| You are here: Home: BCU Surgeons 2004 Vol 3 Issue 3: Peter M Ravdin, MD, PhD
Peter M Ravdin, MD, PhD |
EDITED COMMENTS |
 ADJUVANT! computer program for predicting risk of breast cancer recurrence and mortality ADJUVANT! computer program for predicting risk of breast cancer recurrence and mortality
The ADJUVANT! computer program (available at: www.adjuvantonline.com) is based on about a decade of work that originated with information from the San Antonio database. Originally the underpinnings of ADJUVANT! were based on the Surveillance, Epidemiology and End Results (SEER) database, a large populationbased database with some great strengths. I believe it is a relevant database, and it’s certainly more population-based than the clinical trials in which only about three percent of patients participate. However, some approximations about the impact of adjuvant therapy in different groups must be made because information about them is not included in SEER.
ADJUVANT! focuses on the baseline data for untreated patients and incorporates the Oxford overview data about the efficacy of different adjuvant therapies. The Oxford overview approximates the absolute benefit by multiplying baseline estimates and proportional risk reductions. One of the strengths of ADJUVANT! is its extensive help files which describe the assumptions that underlie some of the estimates. Like the Oxford overview, the assumptions in ADJUVANT! are based as much as possible on global composite information.
Because all of the parameters used in ADJUVANT! and how they were reached are discussed in the help files, if someone disagrees with the proportional risk reductions that were assigned to a particular therapy, their own estimate can be entered.
Information about competing natural causes of mortality is also incorporated into ADJUVANT!. In many of our older patients with node-negative disease, competing causes of mortality may be more important than their risk from breast cancer. To some extent, the urge to treat every patient is predicated on the concept that breast cancer is the only issue for the patient. For many patients with breast cancer, particularly the older ones, the competing causes of mortality reduce the actual benefit and the number of patients with a chance for long-term benefit.
Clinical trial results of adjuvant aromatase inhibitors
Several recent reports offer important data from trials of aromatase inhibitors in postmenopausal women with early breast cancer. The first is the ATAC trial. As first-line adjuvant therapy, anastrozole is about 20 percent better than tamoxifen. In the first public presentation of data from the trial comparing letrozole to placebo after five years of adjuvant tamoxifen, patients receiving letrozole were reported to have a 40 percent proportional risk reduction in relapse events. Like the ATAC trial, the results were presented early because they were extremely positive.
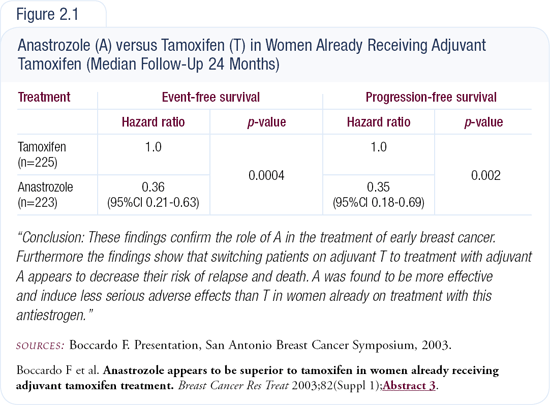
An Italian trial also presented at the 2003 San Antonio Breast Cancer Symposium was very provocative. In that trial, patients received a total of five years of adjuvant endocrine therapy. They all received two to three years of adjuvant tamoxifen and were then randomly assigned to complete their therapy with tamoxifen or anastrozole. The patients who switched to anastrozole had a 60 percent proportional reduction in the risk of relapse, which was greater than expected (Figure 2.1).
Time course for breast cancer recurrences
Because two-thirds of the recurrences occurring within the first 10 years happen in the first five years, the greatest risk of recurrence is during the first five years. There are two potential strategies for adjuvant therapy. The first is to always use the best drugs first because the patients are at the highest risk. The converse would be to use the best drugs later because the impact of stopping tamoxifen is proportionally larger, and that strategy would take a bigger bite out of the late recurrences.
I believe both strategies have a lot of uncertainties, but they may end up being fairly equivalent at 10 years. However, applying the general principle of adjuvant therapy that more benefit may be derived when the best drugs are used first, it appears that starting with an aromatase inhibitor would be the best path.
Use of up-front adjuvant aromatase inhibitors in postmenopausal women
In the nonprotocol setting we inform patients of the continued uncertainties, but we have started treating most postmenopausal women with up-front adjuvant aromatase inhibitors. I don't feel uncomfortable accommodating patients who don't want an adjuvant aromatase inhibitor, because the letrozole study and the Italian study found that switching from tamoxifen doesn't abrogate the positive impact of an aromatase inhibitor. Currently, however, most of my postmeno-pausal patients with ER-positive disease are treated up front with anastrozole.
Role of the aromatase inhibitors following five years of adjuvant tamoxifen
All patients with Stage II or Stage III disease who have recently completed a five-year course of adjuvant tamoxifen should receive an aromatase inhibitor. Whether patients with Stage I disease should receive an aromatase inhibitor is an open question because they have a relatively small amount of residual risk. The aromatase inhibitors can be quite expensive for a fairly marginal benefit in patients with very low-risk disease. Additional costs are associated with monitoring bone mineral density or treating with a bisphosphonate. I would like to see more data in Stage I patients.
Although we don't yet have any data for patients who have finished their five-year course of adjuvant tamoxifen one or two years ago, we will have some data from the patients in the letrozole trial who were taking placebo and then switched to letrozole. In patients who finished a five-year course of adjuvant tamoxifen one year ago, an aromatase inhibitor is strongly justified. On the other hand, for patients who finished a five-year course of adjuvant tamoxifen five years ago, I don't believe an aromatase inhibitor is justified, and gray area exists for those patients who finished a five-year course of adjuvant tamoxifen between one and five years ago.
Select publications
 |
Dr Ravdin is a Clinical Professor of Medicine at The University of Texas Health Science Center at San Antonio in San Antonio, Texas. |
|
