| You are here: Home: BCU 4|2004: Martine Piccart, MD, PhD
Martine Piccart, MD, PhD |
EDITED COMMENTS |
Selecting systemic therapy for patients with an initial relapse
I look at the characteristics of the primary tumor, the duration of the disease-free interval, the sites of the metastases and whether they are symptomatic or life-threatening.
If I am confident there is a reasonable chance that the woman will respond to endocrine therapy, it will be my first choice. I keep women on endocrine therapy as long as possible.
The best way to sequence hormonal agents is not yet known. However, strong evidence from several Phase III trials suggests that an aromatase inhibitor is superior to tamoxifen in postmenopausal women. No reason exists to not use an aromatase inhibitor initially in those women. Ongoing trials will determine the optimal therapy for women with disease that progresses on an aromatase inhibitor.
Role of fulvestrant in patients with disease that progresses on adjuvant tamoxifen
I’m still using an aromatase inhibitor as my first choice. Fulvestrant is a rational choice for patients who have been treated with an aromatase inhibitor and have disease progression. Since the estrogen receptor is still present and possibly hyperactive in these situations, it makes sense to use fulvestrant, which destroys the estrogen receptor. I believe that in the trials comparing fulvestrant to other drugs, fulvestrant will be superior.
In Belgium, fulvestrant is not registered for use, but we are able to obtain the drug on a compassionate-use basis. We have had a very good experience in terms of long-term disease stabilization rather than true objective response. We have been using fulvestrant in women who have received several lines of endocrine therapy.
This experience is encouraging and clearly demonstrates that fulvestrant has the potential to offer some activity, even after two to three lines of prior therapy. We have seen very long-lasting disease stabilization with this extremely well- tolerated agent.
Resection of liver-only metastases in patients with HER2-positive, ER-negative disease
We need to approach patients who have HER2-positive, ER-negative disease with a different mindset. Sometimes women with HER2-overexpressing breast cancer and liver metastases have a dramatic response to a taxane and trastuzumab. After a while, the taxane must be discontinued because of toxicity, but trastuzumab is continued. If the disease is still controlled at that time, the woman will probably be a long-term survivor.
In these situations I consider whether a surgeon should try to remove the remaining metastatic lesions in the liver. Ideally, we should conduct a randomized trial to prove that this strategy will improve survival. However, I have done it in a few select cases — very young women who were willing to fight as much as possible. We discuss that there is no proof that resection of the liver metastases will improve their survival, and I don't propose this approach with only three to four months of treatment. Initially, I observe the quality of the response. I begin to consider this potential strategy when the woman is nine to 10 months from treatment initiation, doing well and has negative imaging studies.
Brain metastases in patients treated with trastuzumab
Approximately one-third of women who are responding to trastuzumab develop brain metastases. A few of my patients with brain metastases have died even though they had a complete remission in the liver. We need to develop clinical trials to address this problem, and I'm hoping that we can find something better than prophylactic cranial irradiation (PCI).
Perhaps it would be best to conduct a study with PCI when we are able to predict which women are at high risk for developing brain metastases. Maybe genetic profiling could help us, and we need to start investigating potential molecular markers that might indicate a higher risk of developing this complication.
Since these are patients in whom the disease is responding beautifully in other sites, it's obvious there is a problem with trastuzumab entering the blood-brain barrier (Figure 2.1). It is possible that the adjuvant trastuzumab trials may demonstrate an improvement in distant disease-free and overall survival but an increased risk of brain metastases. We are going to evaluate that very carefully in the large European adjuvant trastuzumab trial. Brain metastases may also be a problem with the taxanes.
Trastuzumab alone or in combination with chemotherapy
I use trastuzumab alone in a minority of patients — elderly women or young women who are not willing to undergo another course of chemotherapy. Otherwise, I prefer a combined strategy. In patients who have had a prior response to chemotherapy and trastuzumab and are now receiving trastuzumab alone but have disease progression, I don't stop trastuzumab; instead I reintroduce chemotherapy for three to four months. I have seen nice responses in those situations. If the treatment-free interval was long, I might use the initial chemotherapy. If the treatment-free interval was six months or less, I would select a different agent.
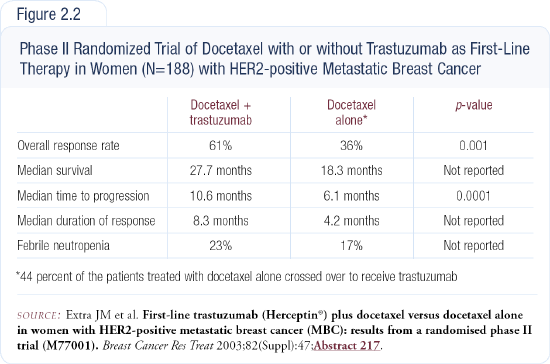
We have strong data supporting the use of taxanes in combination with trastuzumab. A recent trial comparing docetaxel with or without trastuzumab had striking results favoring the combination (Figure 2.2). I believe it is a good regimen to choose. If I were to use paclitaxel, I would administer it weekly with trastu-zumab. The trials comparing vinorelbine and paclitaxel are interesting. I have seen impressive anecdotal responses to vinorelbine plus trastuzumab.

Scheduling of trastuzumab
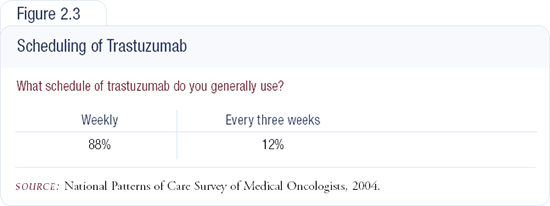
Good data about the every three-week trastuzumab schedule do not exist. A Phase II trial with about 100 patients demonstrated a response rate for every three-week trastuzumab that was similar to the response rate with a weekly schedule. I'm still a little uncomfortable because I do not believe that either of the schedules is the right one, and there could potentially be a better schedule.
Our Canadian colleagues have been evaluating a higher loading dose administered up front. Since I'm still uncomfortable with the question of optimal schedule in a woman at high risk who needs a rapid response, I start with a weekly schedule and then switch to an every three-week schedule as soon as I know she's responding. This is a very conservative approach, and I am not saying I am right. It's just the way I do it (Figure 2.3).
Nonprotocol management of patients with HER2-negative, ER-negative metastatic disease
These patients can only benefit from chemotherapy. I use combination chemotherapy when I need a quick response and sequential single agents when I don't. In a patient who has recently received adjuvant AC and a taxane and has relapsed, I would probably use capecitabine 2,100 mg/m2, two weeks on and one week off, as my first choice for a sequential single agent.
Interestingly, an ongoing EORTC trial (EORTC-10001) is comparing capecitabine and vinorelbine in these women. We don't know which drug is better in this situation, but women tend to like an oral drug and many would choose capecitabine. In a woman who has received prior adjuvant ACT and is ill with metastatic disease, I like to use vinorelbine and capecitabine or vinorelbine and 5-FU regimens, which are quite effective and relatively well-tolerated.
Select publications
 |
| Dr Piccart is the Head of the Chemotherapy Department at the Jules Bordet Institute and Chairwoman of the Breast International Group in Brussels, Belgium. |
|
