| You are here: Home: BCU 4|2004: Maria Theodoulou, MD
Maria Theodoulou, MD |
EDITED COMMENTS |
CALGB-49907: Capecitabine versus CA/CMF in the elderly
This trial evaluates whether there is equivalence between the standard chemotherapy regimens of AC or CMF versus oral capecitabine, and how these regimens impact survival in elderly women, for whom we have very little trial data.
In addition to the more familiar ER, PR and HER2 markers, we are looking at some interesting predictive and prognostic markers and other biological markers. We are also examining how these drugs are processed in the elderly population.
The data from the metastatic setting provided the rationale for selecting capecitabine for this trial. In addition to the convenience of an oral regimen, the trials comparing capecitabine to single-agent paclitaxel and to CMF demonstrated benefits from capecitabine in time to progression. However, capecitabine is not a benign drug, so we are closely monitoring patients.
Originally, the dosing for capecitabine started at 2,000 mg/m2 per day in divided doses, two weeks on and one week off for four cycles, and could be increased to the 2,500 mg/m2 level described in the package insert. However, two deaths have occurred during the trial, one of which was consistent with a DPD deficiency. This patient became myelosuppressed and septic within just a few days of receiving her first cycle. The second patient, who had tolerated most of her treatments very well, was dose-escalated and ultimately developed toxicities and died. As a result, the trial was amended, and capecitabine dosing is now capped at 2,000 mg/m2.
Choice of single-agent versus combination chemotherapy in patients with HER2-negative, ER-negative disease
My approach in selecting single-agent versus combination chemotherapy really depends on whether a patient presents with visceral crisis and a heavy burden of disease. I tend to use capecitabine/docetaxel combination therapy in patients with visceral crises. I don't want to wait two or three months to see what kind of response the patient will have with single-agent therapy (Figure 4.1). I'm impressed by the fast responses with the combinations.

If, on the other hand, I have an asymptomatic patient with low-burden disease, I will stretch my single-agent therapy in a sequential fashion for as long as I can. The sequence will depend upon the treatment goals I have established with the patient, what toxicity profile the patient is willing to undertake and the disease-free interval from the most recent chemotherapy. If, for example, a patient relapses within six months of receiving AC followed by docetaxel, I would opt for a non-taxane-containing regimen.
In stable patients I would probably use capecitabine because of its oral administration and excellent response profile. In patients who progress on capecitabine, I would look at other single agents, such as vinorelbine or gemcitabine, with the order depending on what side effects patients were willing to tolerate.
In patients presenting de novo with HER2-negative metastatic disease, I will opt for capecitabine/docetaxel, depending on their burden of disease. If I want a quick response, I'll use anthracyclines up front, knowing that I have a finite window of opportunity for treatment. In patients with ER-negative disease I generally prefer drugs that I'll be able to use long-term.
Clinical trial evaluating nonpegylated liposomal doxorubicin plus trastuzumab
The pivotal trial of chemotherapy and trastuzumab demonstrated a 27 percent overall and 16 percent symptomatic cardiac dysfunction rate, but the trial also demonstrated survival benefits in the anthracycline/trastuzumab arm. This inspired us to find a safer way to bring this class of agents back into the clinical setting with trastuzumab. We are particularly motivated because trastuzumab is now being evaluated in the adjuvant setting, and we'd like to identify a cardiac-safe regimen for potentially curable patients.
Gerald Batist looked at this issue in a trial of liposomal versus conventional doxorubicin plus cyclophosphamide, which demonstrated equivalent time to progression in both arms and a cardiac safety advantage in the liposomal arm. We participated in a cardiac safety trial of nonpegylated liposomal doxorubicin in combination with trastuzumab.
We studied 37 women with this drug combination and saw that only two patients actually had cardiac toxicity. One patient was asymptomatic but was withdrawn because of a drop in LVEF; the other patient had symptomatic cardiac disease. The low rate of nonhematologic toxicity was wonderful, but the real surprise in the trial was the 58 percent overall response rate and a clinical benefit of 79 percent.
These exciting results may encourage further study of liposomal doxorubicin in combination with trastuzumab. Jose Baselga, for instance, is leading a European team of investigators in studying the metastatic application of this drug combination with a taxane in patients with HER2-positive disease.
Treatment of HER2-positive, anthracycline-naive patients with metastatic disease
I'll treat these patients with trastuzumab plus vinorelbine or a taxane, or use a triplet such as carboplatin/paclitaxel/trastuzumab, depending upon the patient's tolerance for side effects. Of course, it would be ideal to enroll patients in a clinical trial with trastuzumab and liposomal doxorubicin. I withhold anthracyclines until later.
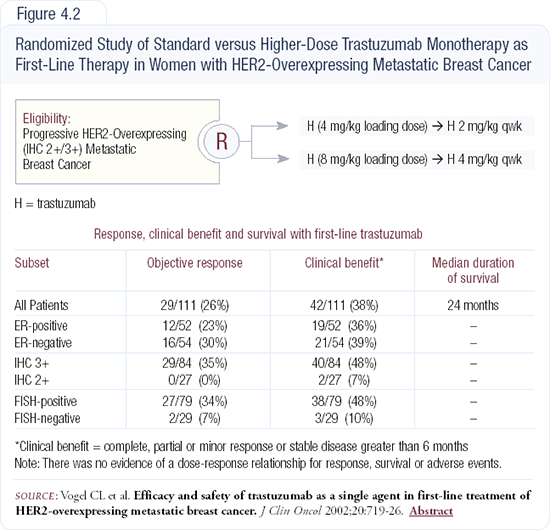
Trastuzumab monotherapy is also a reasonable option for patients with small-volume, HER2-positive disease who are not open to the idea of chemotherapy. Chuck Vogel demonstrated a 47 percent clinical benefit with trastuzumab monotherapy in chemotherapy-naive patients with measurable metastatic disease (Figure 4.2). I tend to use trastuzumab with chemotherapy up front and then apply trastuzumab alone as maintenance treatment.
Choice of chemotherapy in combination with trastuzumab for metastatic disease
I have been using carboplatin/docetaxel/trastuzumab frequently, especially in patients with bulky disease and visceral crises. My choice of which chemotherapeutic agent to use is guided by the toxicities a patient is willing to tolerate. A woman with newly diagnosed metastatic disease may feel absolutely violated by the idea of hair loss with the use of a weekly taxane. I also like the vinorelbine/trastuzumab combination. It's well-tolerated and generates good responses.
Once a patient reaches an optimal response on combination therapy, I discontinue the chemotherapy and maintain them on trastuzumab almost indefinitely. Some of my patients have been on monotherapy for three or four years, if only to avoid the possibility of upregulating proliferative mechanisms when trastuzumab is stopped.
If patients progress on maintenance trastuzumab, I go back to the original chemotherapy/trastuzumab regimen and often I've regained a response. If I do not regain a response, I quickly switch to a different chemotherapy after eight to 10 weeks. Even when I switch chemotherapies, I continue the trastuzumab as long as I'm getting a response from the new chemotherapy.
If a patient progresses rapidly after introducing a new chemotherapy agent, it becomes apparent that trastuzumab is no longer effective. At this point, I stop the antibody and look for available clinical trials or introduce conventional anthracy-clines in patients who have never had them. If a patient has tried the conventional anthracyclines, I use weekly anthracyclines.
Algorithm for HER2 testing
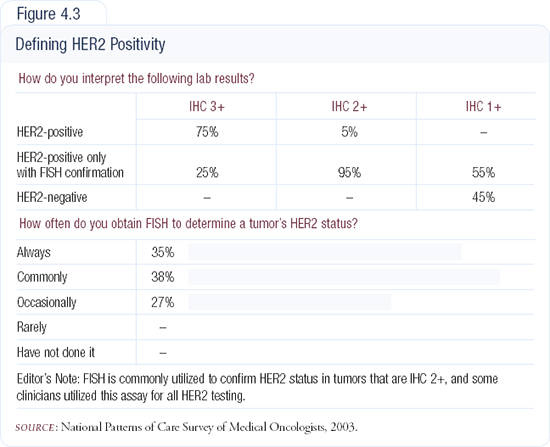
A forthcoming publication will provide the results of an analysis of tissue samples we conducted at Memorial Sloan-Kettering. We evaluated almost 3,000 surgical specimens. Of those samples that received a zero grading by good pathologists and a good reference lab with large volume experience, using the HercepTest®, we tried to determine how many were actually FISH-amplified. We found that one in 100 of our specimens was FISH-amplified, despite having tested zero by IHC.
Our analysis also demonstrated that some IHC 1+ samples were FISH-amplified. We know that approximately 10 percent of 1+ patients will show FISH-amplification. Although in most clinical settings an IHC of zero to 1+ is deemed negative for HER2, we occasionally encounter a patient who is FISH-amplified (Figure 4.3).
A retrospective analysis of 500 paraffin blocks demonstrated that patients who scored zero or 1+ were FISH-amplified three and seven percent of the time, respectively. In patients who were IHC 2+, 25 percent were FISH-amplified. IHC 3+ cases correlated with FISH over 90 percent of the time.
Clearly, there remains a gray area surrounding IHC scores of 1+ and zero. This has a huge impact in the metastatic setting. You may treat a patient with a non-trastu-zumab-containing regimen who is refractory to chemotherapy and not responding to hormonal therapy the way in which you would expect most metastatic breast cancers to respond. In patients who have a short disease-free survival and present with metastatic disease fairly quickly after adjuvant treatment for node-negative disease, I often go back and check for gene amplification with FISH.
CALGB-9741: Dose-dense versus conventionally scheduled chemotherapy
The dose-dense study recently reported by Marc Citron was very interesting. It attempted to answer the question of how to apply mathematical modeling in our efforts to circumvent chemotherapy resistance in the adjuvant setting, while simultaneously optimizing survival.
Specifically, the study examined chemotherapy regimens given every 14 days with growth factor support, as opposed to the conventional 21-day cycle. What I found most impressive was the safety data that showed the very minimal toxicities associated with the dose-dense therapy. Whether the survival curves continue to separate remains to be seen.
Although confirmatory trials are needed to determine the potency of this regimen, dose density might prove to be the way to treat patients in whom cell mutation is a concern. Could we be over-treating patients with this regimen? Perhaps, but given the safety data so far reported, I'm very enthusiastic about using dose-dense chemotherapy and would be comfortable with that "over-treatment" in a node-positive patient.
Select Publications
 |
| Dr Theodoulou is an Associate Attending Physician at the Memorial Hospital for Cancer Diseases in New York, New York. |
|
