 |
HER2 Status and Response To Trastuzumab |

Objectives |
-
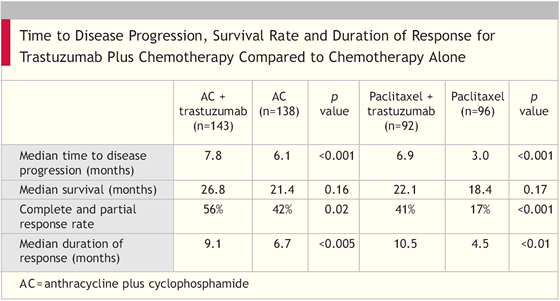
To compare the time to disease progression, incidence of adverse effects, rates and duration of responses, time to treatment failure, and overall survival for chemotherapy plus trastuzumab and chemotherapy alone in women with metastatic breast cancer
|
Eligibility |
- Metastatic breast cancer, previously untreated with chemotherapy
- HER2 2+ or 3+ measured by the Clinical Trial Assay (CTA)
|
Schema |
ARM 1: Chemotherapy* q 3 weeks x 6
ARM 2: Chemotherapy* q 3 weeks x 6 plus trastuzumab q week |
*Chemotherapy = AC, if no prior adjuvant anthracycline, or paclitaxel if patient received prior adjuvant anthracycline-based chemotherapy
Trastuzumab was continued until disease progression. Upon disease progression, 66 percent of the women elected to receive trastuzumab alone or in combination with other therapies.
Authors’ Conclusions
“We found that trastuzumab-based combination therapy was effective in that it reduced the relative risk of death by 20 percent at a median follow-up of 30 months.... Particularly noteworthy is that two-thirds of patients who were initially assigned to receive chemotherapy alone began, after disease progression, to receive open-label trastuzumab alone or with chemotherapy. Such a crossover design would generally reduce the likelihood that a survival advantage would be found. Significant increases in the time to disease progression, the rates of response, the duration of responses and the time to treatment failure were observed in both subgroups that were given chemotherapy plus trastuzumab. These results increased survival, an end point free of ascertainment bias.”
| Research Leader Commentary |
We used a combination of an anthracycline and cyclophosphamide, which was commonly used as first-line therapy in metastatic disease. For those patients who had received adjuvant doxorubicin, paclitaxel was utilized. Essentially, the patients were randomized to the best available standard chemotherapy plus or minus trastuzumab.
In the Phase III trial, the addition of trastuzumab led to a significant improvement in response rate, response duration and time to progression. A little-known fact from that trial is that the highest response rate was seen in the anthracycline/cyclophosphamide and trastuzumab arm. Paclitaxel/trastuzumab was ultimately included in the package label because of the toxicity encountered with the other arm.
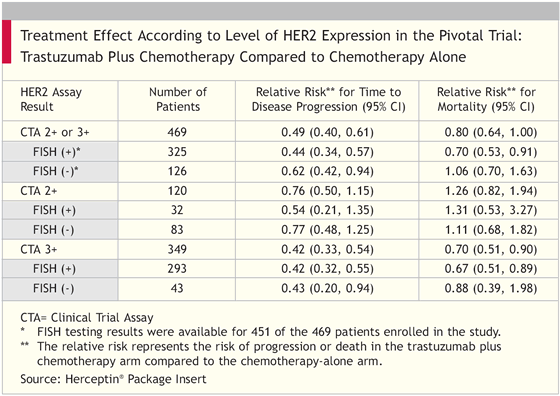
We were very encouraged with the improvement in the median time to progression for the group receiving trastuzumab. Although it is only a three-month improvement, it translates, ultimately, into a survival advantage. At four years of follow-up, trastuzumab decreases the relative risk of death by 30 percent in women with truly HER2-positive breast cancer — those which are FISH-positive.
Dennis Slamon, MD, PhD |
Page 1 of 6
Next
|
|