|

Objectives |
- To assess the safety and response rate of trastuzumab plus docetaxel in women with HER2-overexpressing metastatic breast cancer
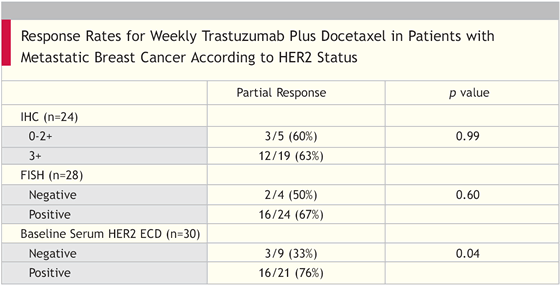
- To evaluate the role of circulating HER2 extracellular domain (ECD) concentration as a predictor of response to trastuzumab therapy
|
Eligibility |
- Metastatic breast cancer previously treated with no more than three chemotherapy regimens
- HER2-overexpression assessed by IHC with the monoclonal antibody, e2-4001, or by FISH with the PathVysionTM FISH assay
|
Schema |
(Trastuzumab + docetaxel) q week x 3 every 4 weeks
|
Treatment was continued until disease progression or the appearance of prohibitively toxic effects.
- The estimated median time to progression was nine months.
- There was a longer time to progression in patients receiving weekly trastuzumab plus docetaxel as first-line therapy than in patients receiving it as second-line therapy.
Authors’ Conclusions
“Weekly administration of docetaxel and trastuzumab is safe and effective for patients with metastatic breast cancer whose tumors overexpress HER-2. Further research is warranted to determine the value of serum HER-2 ECD testing in selecting and monitoring patients undergoing trastuzumab-based therapy.”
Page 4 of 6
Previous | Next
|