
In view of the new data from
the ATAC trial, should anastrozole now be used as adjuvant therapy
for postmenopausal women with early invasive breast cancer?
 |
|
OVERVIEW:
Tamoxifen has been the predominant form of
adjuvant endocrine therapy since the first International
Breast Cancer Overview was presented at the 1985 NIH
Consensus Conference. Currently, most patients with
invasive estrogen receptor-positive cancers are treated
with tamoxifen regardless of age, menopausal status
and risk to recur. On December 10, 2001, the initial
results of the ATAC trial were presented at the San
Antonio Breast Cancer Symposium. These groundbreaking
data demonstrated that in postmenopausal women with
primary invasive breast cancer, the third generation
aromatase inhibitor, anastrozole, conferred an advantage
over tamoxifen in terms of efficacy, tolerability
and toxicity. No advantage was observed to combining
anastrozole with tamoxifen. Clinicians and patients
are now struggling with the clinical implications
of these early but very promising results from the
largest cancer treatment trial ever conducted.
|
|
 |
 |
| |
Which of the following describes how you generally
identify a breast cancer as being ER-positive?
| DISEASE |
|
ANY STAINING
|
STAINING ABOVE A SPECIFIC
CUT-OFF AS DEFINED BY
THE LAB DOING THE TEST
|
ER-positive
breast cancer
|
50%
|
50%
|
Which adjuvant endocrine therapy would you
recommend for the following patients with ER-positive, HER2-negative
breast cancer?
| |
TAMOXIFEN |
ANASTROZOLE
|
LETROZOLE |
NONE/OTHER |
|
|
65-year-old
woman with a 2.2 cm tumor/10+ nodes
|
60%
|
30%
|
5%
|
5%
|
| 65-year-old
woman with a 2.2 cm tumor/2+ nodes |
50%
|
40%
|
5%
|
5%
|
| 65-year-old
woman with a 2.2 cm tumor/neg nodes |
55%
|
35%
|
5%
|
5%
|
| 65-year-old
woman with a 0.8 cm tumor/neg nodes |
35%
|
35%
|
10%
|
20%
|
| 77-year-old
woman with a 2.2 cm tumor/10+ nodes |
50%
|
40%
|
10%
|
0%
|
| 77-year-old
woman with a 0.8 cm tumor/neg nodes |
35%
|
15%
|
5%
|
45%
|
How would you manage the following patients
with ER+, HER2-negative breast cancer?
| |
REFER TO
ONCOLOGIST
|
START
TAMOXIFEN
|
START
ANASTROZOLE
|
MANAGE WITHOUT
ADJUVANT SYSTEMIC
THERAPY
|
MANAGE PT.
PRIMARILY WITH
TAMOXIFEN
|
|
|
65-year-old
woman with a 0.8 cm tumor/neg nodes
|
50%
|
5%
|
40%
|
5%
|
5%
|
| 65-year-old
woman with a 2.2 cm tumor/neg nodes |
75%
|
10%
|
10%
|
5%
|
-
|
| 65-year-old
woman with a 1.2 cm tumor/1+ node |
85%
|
5%
|
10%
|
-
|
-
|
| 77-year-old
woman with a 0.8 cm tumor/neg nodes |
50%
|
-
|
30%
|
5%
|
15%
|
Which of the following best describes your
intended use in the near future of aromatase inhibitors as adjuvant
therapy?

If the ATAC data are widely accepted, and anastrozole
generally replaces tamoxifen as adjuvant endocrine therapy for
postmenopausal women, how likely is it that surgeons will prescribe
anastrozole?
Surgeons
will prescribe
anastrozole |
VERY LIKELY
|
LIKELY
|
SOMEWHAT LIKELY
|
VERY UNLIKELY
|
UNDETERMINED
|
|
30%
|
25%
|
25%
|
15%
|
5%
|
SUMMARY OF ATAC RESULTS
The headline news is that there is something after tamoxifen
— there is a significant advantage for anastrozole compared
to tamoxifen. The real surprise is that the combination of anastrozole
and tamoxifen is no different than tamoxifen alone. What makes
these early ATAC results even more extraordinary is that about
15% of the trial population was ER-negative and ER unknown. When
you look at the analysis of the known ER-positive patients, the
results are even stronger. The hazard ratio for anastrozole compared
to tamoxifen is 0.78. This is equivalent to a 22% relative reduction
in risk of recurrence compared to tamoxifen.
—Michael Baum, ChM, FRCS
OUTCOMES OF THE ATAC TRIAL
I think it’s very surprising that the benefit of anastrozole
emerged so clearly and so strongly. We’re talking about almost
a 25% reduction in recurrence compared to tamoxifen in estrogen
receptor-positive women. Tamoxifen itself produces about a 40%
reduction in recurrence rates, so to get another 25% on top of
that is really quite substantial. Even more, we are seeing these
results with a side effect profile that is generally more favorable.
However, these are the early days for a new drug that produces
very, very low estrogen levels. Clearly, these low levels affect
the bones and there is concern that they might affect memory and
cognitive function as well. These are probably manageable problems,
but this is one of the areas where we need to be cautious.
—Jack Cuzick, PhD
SIDE EFFECTS AND TOXICITIES WITH TAMOXIFEN
AND ANASTROZOLE
ATAC demonstrated that fewer side effects are associated with
anastrozole than tamoxifen. Thromboembolic complications, vaginal
bleeding, spotting and discharge, and endometrial cancer were
all substantially lower in the anastrozole arm.
In terms of the side effects seen with anastrozole, I have not
seen any patient in which arthralgias forced us to change or stop
therapy. The potential negative effects on bone of anastrozole
can be watched very closely. If there is a change in bone density,
highly-effective interventions can be pr ovided.
—Aman Buzdar, MD
SUBSTITUTING OTHER AROMATASE INHIBITORS IN
THE ADJUVANT SETTING
There are no data to support using letrozole or exemestane in
the adjuvant setting. Since anastrozole is the only drug that’s
been tested, it is the drug we should use. All of the aromatase
inhibitors are slightly different. We need direct comparative
data for these drugs. If they are equally effective, it might
come down to which one has the fewest side effects and best tolerability.
Until we have comparative data, the drug which has been tested
in the adjuvant setting should be used.
—J Michael Dixon, MD, FRCS
ADJUVANT ENDOCRINE TREATMENT: IMPLICATIONS
OF ATAC
In the evolution of science and medicine there are periods of
uncertainty. We are living in such a time now. If the efficacy
advantage for anastrozole continues, then we can start making
therapeutic recommendations. Presently we have only two and one-half
years of treatment data. We cannot be certain about what will
happen with further therapy.
Newly diagnosed women should be informed of the ATAC data in
a responsible way. Most of them will make a rational decision.
I believe that tamoxifen should continue to be considered the
gold standard, at least until the trial results are updated at
ASCO 2002. However, my colleagues have been putting some adjuvant
patients on anastrozole on the basis of the positive findings
in the metastatic setting — without the adjuvant data —
and it is a legitimate non-protocol option where there are contraindications
to tamoxifen. If women are already on adjuvant tamoxifen, it would
be hazardous to switch to anastrozole, since we haven’t tested
that sequential therapeutic rapproach.
—Michael Baum, ChM, FRCS
The only shortcoming of the ATAC data is the relatively short
follow-up period. Of course, everyone would like to see drugs
followed for 20-30 years, but we can’t decide the fate of
the patients while waiting for the data to become that mature.
In the ATAC trial, there is a significant reduction in the risk
of recurrence and fewer side effects and fewer potentially lethal
complications associated with anastrozole compared to tamoxifen.
It is my responsibility as a physician to share information with
patients and let them be active participants in making their own
decisions. However, if I were a patient, I would opt for anastrozole
even though there is a short period of follow-up.
—Aman Buzdar, MD
| 3RD GENERATION
AROMATASE INHIBITORS |
|
|
 |
|
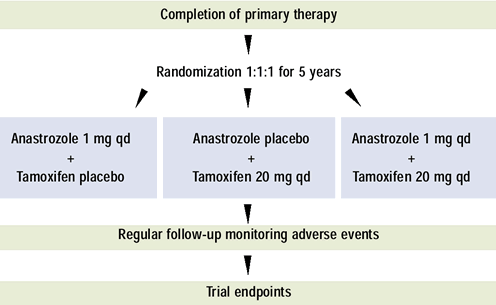
ATAC TRIAL - STUDY ENDPOINTS |
|
PRIMARY ENDPOINTS
- Disease-free survival
- Locoregional or distant recur rence, new primary
breast cancer, or death from any cause
- Safety/Tolerability
SECONDARY ENDPOINTS
- Incidence of new breast (contral ateral) primaries
- Time to distant recurrence
- Survival (data will be mature in » 2 years)
- Hormone rece ptor-positive population (protocol-defined
sub-group)
|
|
 |
| ATAC
TRIAL - PATIENT CHARACTERISTICS* |
- Mean age in the anastrozole arm was 64.1 years
• 83.7% of patients
in the anastrozole arm were ER-positive
• 47.8% of patients
in the anastrozole arm were treated with mastectomy
• 22.3% of patients
in the anastrozole arm were treated with chemot
herapy
*patients in all arms were similar
|
|
 |
| ANASTROZOLE’S
PROFILE |
- Highly selective, potent aromatase inhibitor
- Non-steroidal
- Orally active (1 mg)
- Once-daily dosing
- Superior to tamoxifen in postmenopausal women
with estrogen receptor-positive advanced breast
cancer
- Survival advantage vs. megestrol acetate in metastatic
disease
- Over 460,000 patient-years experience
|
|
 |
ATAC
TRIAL DESIGN - POSTMENOPAUSAL WOMEN WITH
INVASIVE BREAST CANCER |
|
|
|
 |
| SUBPROTOCOLS
OF THE ATAC TRIAL |
- Hormone rece ptor-positive population (protocol-defined
sub-group)
- Pharmacodynamic and pharmacokinetic profiles
- Modulation of lipoprotein profiles
- Endometrial status
- Bone mineral metabolism
- Quality of life
|
|
| ON-GOING RANDOMIZED
TRIALS OF POST-TAMOXIFEN AROMATASE INHIBITORS IN POSTMENOPAUSAL
BREAST CANCER PATIENTS |
|
Post-Tamoxifen
Treatment
|
Adjuvant
Tamoxifen
(years)
|
Projected
Accrual
|
Sponsor
|
Letrozole
5 years |
4.5 - 6
|
4,800
|
Canadian NCI,
NCCTG, & SWOG
CALGB, EORTC
|
Exemestane
2 years |
5
|
3,000
|
NSABP
|
Exemestane
2 - 3 years |
2 - 3
|
4,400
|
Breast
International Group
|
Anastrozole
3 years |
5
|
1,700
|
Austrian Breast
& Colon Cancer
Study Group
|
TRIALS OF AROMATASE
INHIBITORS AFTER TAMOXIFEN
We've got adjuvant studies that look at aromatase
inhibitors given after tamoxifen. The question now
is, a re those studies going to be re l e vant anymore?
Is it really the studies that use aromatase inhibitors
up front that are going to be the ones we're interested
in? We may have a whole group of large studies with
interesting information that we may not use, because
we won’t be giving the drugs that way anymore.
—Kathleen Pritchard ,M D
|
|
 |
| SUMMARY
OF ATAC TRIAL OUTCOMES |
|
9,366 evaluable patients
- Atamedian treatment duration of 2.5 years
, anastrozole demonstrated superior
efficacy and tolerability compared to tamoxifen
- Anastrozole was superior to tamoxifen in
terms of disease-free survival in the overall
population (relative reduction of 17%) and
in estrogen receptor-positive patients (relative
reduction of 22%)
- Anastrozole was superior to tamoxifen in
terms of the incidence of contralateral breast
cancer in the overall population (relative
reduction of 58%)
- There were 156 patients with distant metastases
in the anastrozole arm and 181 in the tamoxifen
arm (not statistically different )
• There were only a total of five breast
cancer deaths in the three treatment arms
|
|
Anastrozole was tolerated better than tamoxifen
with respect to:
- Endometrial cancer
- Vaginal bleeding
- Vaginal discharge
- Is chaemic cerebrovascular events
- Venous thromboembolic events
- Hot flashes
- Weight gain
|
|
Tamoxifen was tolerated better than anastrozole
with respect to:
- Musculos keletal disorders (arthralgias)
- Fractures
|
|
Derived from a presentation by Michael Baum,
24th Annual San Antonio
B reast Cancer Symposium
Baum M. The ATAC (Arimidex, Tamoxifen, Alone
or in Combination) adjuvant breast cancer trial
in postmenopausal (PM) women. Breast Cancer
Res Treat 2001; 69(3):Abstact.
|
|
|
View References
Top of Page | Back
to Index | Go to Section 2




