|
| Radiofrequency ablation of primary breast
cancer Rache M. Simmons, MD, FACS, Associate Professor of Surgery,
Strang Weill-Cornell Breast Center, New York Presbyterian Hospital,
Weill Medical College of Cornell University, New York, NY |
 |
|
|
|
Radiofrequency Ablation
|
|
Radiofrequency Ablation
|
 |
• Established as effective treatment of metastatic
hepatic tumors
• Experimental for treatment of lung, bone, brain, kidney,
prostate tumors
• Current protocol for treatment of breast cancers radiofrequency
interstitial tissue ablation |
|
• Destruction of solid tumors through application of
high frequency alternating current
• Electrode itself is not the source of heat
• Frictional heat from ions within tissue changing direction
with alternating current
|
 |
|
RF Breast Cancer Protocol
|
|
RF Breast Cancer Protocol
|
 |
• 15 g hollow needle probe
• Multiple electrodes form a star-like destructon
• Temperature sensing probes for feedback to assess target
temperature |
|
• 5-7 minute to reach target temperature
of 95oC (cell death at 50oC)
• 15 minutes at target
• 1 minute cool down |
 |
|
RF Breast Cancer Protocol
|
|
RF Breast Cancer Control
|
 |
• Tri-institutional Trial (NYPH, MD Anderson,
John Wayne)
• Ablate and reset to confirm tumor destruction |
|
• Tumor 2 cm or less in diameter
• Tumor sonographically detectable
• Distance of 5mm from the chest wall and overlying skin
• Preoperative core biopsy diagnosis of invasive carcinoma
and determination of ER/PR |
 |
|
RF Breast Cancer Protocol
|
|
RF Breast Cancer Protocol
|
 |
• At time of breast cancer surgical treatment
• Sedation or general anesthesia
• Sentinel node biopsy (+/- axillary dissection), BMA
• Ablative procedure
• Followed by standard lumpectomy or mastectomy |
|
• Pathological Analysis
• Standard H&E
• NADH tumor viability stain
• Viable cell stain blue (cytoplasmic granules with NADH
oxidation reaction)
• Non-viable cells no stain |
 |
|
Conclusions
|
|
Conclusions
|
 |
• RF offers an exciting potential treatment
for selected breast cancers
• More data is needed to revise patient selection |
|
Proposed next phase of protocol:
• Ablation with leaving tumor in vivo 306 months prior
to resection
• Proceed with adjuvant therapy
• Monitor potential enhanced immunologic response to tumor
• Follow probably involution of tumor |
 |
| Laser Photocoagulation of Breast Cancer
Using MRI Steven E. Harms, MD, FACR, Professor of Radiology,
University of Arkansas for Medical Sciences, Little Rock, AR,
and Medical Director, Aurora Imaging Technology, North Andover,
MA |
 |
Introduction
Breast conservation surgery was developed to reduce the disfigurement
of mastectomy while providing an equivalent therapeutic outcome.2,3,4,12,50,62
Most women now prefer lumpectomy to mastectomy surgery, despite
the increased cost and need for radiation therapy. These factors
indicate the value our society places on improved cosmesis. Small
breast cancers (< 1 cm) have an excellent prognosis, with a disease-free
survival at 20 years approaching 90%.27,33,37,38,48,56 These outstanding
results were derived from long term follow-up studies where breast
conservation was not yet available. Since these patients were treated
with mastectomy, it is clear retrospectively that they achieved
little therapeutic gain from the loss of their breast.
Following the philosophy of breast conservation therapy, the obvious
next step is to apply modern minimally invasive methods to achieve
an even greater cosmetic benefit. Minimally-invasive therapy has
been applied for a variety of solid tumors in other organs including
liver, brain, prostate, lung, pancreas, and uterus.1,6,32,39,40,43,51,60,61
These methods effectively destroy tissue, and the application for
the destruction of breast tumors is straightforward19,20,25,26,42,47.
Despite the potential importance of minimally invasive therapy
in the breast, its application has been more cautious than in other
systems. There are several reasons for concern on the part of research
teams in this area. The use of minimally invasive therapy in most
current applications involves palliation of disease where the therapeutic
alternatives incur more risk or are not available. For example,
the treatment of colorectal metastases to the liver can improve
an otherwise dismal prognosis. There is little downside risk to
undergoing the minimal-risk procedure. The alternative to the treatment
of liver metastases is surgery, where the risk of death is significant.
The use of minimally invasive therapy for breast cancer, however,
is considered in a completely different clinical picture. Patients
with small breast cancers that are applicable to minimally invasive
therapy already have an outstanding prognosis. If the treatment
fails, they may have lost their best chance for curing a clearly
treatable disease. These ethical concerns will be some of the greatest
challenges in the development of clinical trials for minimally invasive
therapy in the breast.
This chapter will outline the integration of breast MRI and minimally-
invasive therapy for breast tumors, considering the various technical
obstacles that are typically encountered.
How do we know if we are treating all of the disease?
It is well known that subclinical residual disease is present after
lumpectomy surgery. A number of rigorous pathologic studies using
serial sectioning of mastectomy specimens have documented the occurrence
of otherwise unsuspected foci of disease in about 40% of breasts.
30,35,55 In addition, the NSABP B-06 trial determined that a similar
percentage of cancers would recur if radiation therapy was not given.
These findings are the justification for the routine use of radiation
following lumpectomy surgery.12 It is clear that radiation will
be needed in patients treated with minimally invasive therapy. More
importantly, the therapeutic benefit of radiation is only achieved
when the pathologic margins of the lumpectomy are clear of residual
tumor.15,46,49,52,54,59
Validation of clear pathologic margins will not be possible when
minimally- invasive therapy is employed. This would not be an issue
if we knew that our imaging methods were accurate in the definition
of tumor margins. Unfortunately, clinical trials have proven the
traditional methods for predicting margins are very inaccurate.
About half of lumpectomy surgeries can be expected to reveal positive
margins upon pathologic analysis.15,46,49,52,54,59 One Japanese
study determined that 90% of simulated lumpectomies would result
in residual tumor.17 Due to the high incidence of positive pathologic
margins, we need a greater certainty of a potential for clear margins
than is currently available from conventional imaging methods.
Can MRI be used to reliably determine clear treatment margins?
If we no longer have pathology to determine adequate lumpectomy
margins, we will need imaging guidance that will be equivalent to
the accuracy of pathology. Most published MRI series have not been
validated with this feature in mind. The objective for most MRI
studies was to determine the benign from malignant.28,44 Areas of
the breast that are thought to be negative by imaging are not systematically
sampled. This design leads to an under-reporting of false negatives.
There has been little effort directed toward the validation of MRI
as a method to achieve clear pathologic margins. Therefore, the
direct application of most MRI methods for minimally invasive therapy
of breast neoplasms is not straightforward. The need for accurate
margin determination was a major incentive for the validation of
RODEO (Rotating Delivery of Excitation Off-resonance) as a staging
method for breast cancer. This analysis was performed by systematically
correlating RODEO images with serial sectioned mastectomy specimens.
The specimen sectioning followed previous pathologic studies where
the breast was sliced at 5 mm intervals, mapped by the pathologist,
and correlated with the MRI findings. This approach allowed the
accurate evaluation of lesion sizes and margins and provided potential
for determining the prevalence of false negatives. 8,21,22,23,24
DCIS is commonly encountered and is important in the management
of breast cancer. Pure DCIS may represent up to one third of cancers
in some unique circumstances that must be considered for breast
lesion therapy. Many of these therapies have been applied to the
treatment a screened population and commonly accompanies infiltrating
cancer. Its accurate demonstration is important for treatment, as
it must be adequately excised to achieve optimal therapeutic benefit.
Recent studies have demonstrated the difficulty in determining the
presence of DCIS with dynamic low resolution MRI. 9,34 This may
be attributed to the larger voxels needed to achieve a higher acquisition
speed, which results in greater volume averaging effects. This problem
can be corrected with higher spatial resolution and higher contrast.
RODEO has been shown to reliably detect DCIS and characterize microcalcifications.57
We are now employing RODEO to localize margins of DCIS prior to
excision. RODEO can accurately demonstrate the extent of DCIS.
What special considerations are needed for localization methods
when minimally invasive therapy is used?
One of the major issues for breast MRI localization procedures
is the “vanishing lesion” problem. The optimal contrast
between tumor and parenchyma occurs at about two minutes after a
bolus of gadolinium contrast. After about five minutes, the lesion
begins to vanish into the background and is no longer visible. This
effect virtually eliminates any form of real-time localization since
the lesion will disappear so quickly.
A variety of stereotaxic methods have been employed successfully
for biopsy and localization. However, some special problems will
be encountered for when minimally invasive therapy is used.
Most MRI localization methods are extensions of a mammographic
technique. Typically, a mammographic localization attempts to place
a single wire through the center of a lesion prior to excision.
Precision is not a major problem as long as the wire is reasonably
close. The location of the wire relative to the lesion can be demonstrated
on a mammogram obtained after the localization procedure.
MRI-directed treatment applications will require considerably more
precision. The vanishing lesion problem means that repeat imaging
after the procedure will not be possible. Accurate needle placement
within and around the lesion is required to define the treatment
field to include the lesion and an appropriate margin.
Real-time needle placement in open MRI systems has been effectively
used for a variety of interventional purposes.10 This procedure
may be useful for some breast lesions such a fibroadenomas that
are well visualized on non-contrast images. For most small breast
cancers, however, real-time localization in an open MRI is not likely
to be effective since confirming images cannot be employed to validate
appropriate needle placement. The vanishing lesion effect has the
greatest impact on this approach. Another problem with the open
MRI systems is lower overall imaging performance that makes definition
of small lesions and their margins more difficult. Perhaps if longer
acting contrast agents become available, real-time positioning would
be become advantageous.
Frameless stereotaxic systems are widely used for head and neck
applications.11 These systems use image data gathered with fiducial
marks that are registered with a computer display to allow an interactive
display with previously acquired images during the interventional
procedure. Typically a high resolution image set is acquired and
loaded into the machine. The patient is taken to the operating room,
where the images guide the procedure without rescanning. This method
works best when rigid, immovable structures such as bones are imaged.
Because of the lack of structure in the breast, deformation of the
breast during the procedure is a significant problem, which is addressed
by some form of compression. One group has successfully used thermal
setting plastic similar to the molds used in radiation therapy to
form cast around the breast in order to maintain a fixed position
during the procedure.11
The most common approach to stereotaxic breast MRI localization
employs breast compression and a needle guide. A common needle guide
uses a plate with multiple holes that are drilled at appropriate
intervals within the plate. The best hole is selected on the MRI
image and the needle is inserted through the hole a measured distance
to the lesion. Another popular approach employs a needle holder
to guide the needle though a window in the plate. Coordinates for
the needle holder are obtained from the MRI image. Both of these
approaches define a unique pathway to the lesion and are most typically
used for single needle localizations or biopsy.13,14,29,31,45,53
These systems are not designed to approach multiple targets from
the same entry or the same target from multiple entries.
Our group recently developed another approach to MRI guided needle
placement that combines some of the attributes of freehand approaches
with stereotaxic systems. Compression plates are drilled throughout
with 1 cm holes so that access to the entire breast is possible.
In addition, the protrusion of the breast through the holes grabs
the skin and provides additional stability to prevent the breast
from rolling when pressure is applied. Guidance is provided indirectly
with a laser beam that is located at some distance from the field.
The best access hole is selected on the MRI image. The distance
from the hole to the lesion and two angles are obtained from the
MRI. The angles are dialed into the laser positioner and the beam
is centered on the selected hole. The tip of the needle is placed
on the laser spot on the skin and the hub is aligned with the beam.
As the needle is guided by hand, tactile sense is preserved and
the trajectory can be tested intermittently by releasing the hub.
If the hub bounces off the beam, the needle is bending off the trajectory.
We have found the deflection of MRI needles to be a particular
problem. This system allows an easy correction for needle deflection.
Multiple needles with different targets can be placed through the
same entry or different entry sites can be selected with the needles
converging on the same target. The latter approach can be used to
simultaneously laser treat a lesion with multiple needles while
thermally isolating the entry sites.7
What kinds of minimally invasive treatment methods are applicable
to the breast?
Minimally invasive treatment methods typically employ some form
of thermal injury that can be delivered with imaging guidance. There
are some unique circumstances that must be considered for breast
lesion therapy. Many of these therapies have been applied to the
treatment of lesions such as metastatic colorectal cancer to the
liver, where margin definition of the lesion is straightforward.
As mentioned previously, breast cancer margins are difficult to
determine and present perhaps the greatest challenge for imaging.
In addition, inadequate treatment of the margin will adversely affect
prognosis. Therefore, coordination of treatment delivery and margin
definition is required for an effective minimally invasive breast-cancer
treatment.
Cryotherapy uses a system that delivers the freezing effect of
liquid nitrogen to a small area. Cryotherapy is now a popular method
for the palliative treatment of liver lesions, particularly colorectal
metastases.36 The size of the probe for cryotherapy is large and
is usually applied with a surgical exposure. Freezing is not as
effective at tumor ablation as hyperthermia. Some tumor cells may
survive freezing. The treatment zone of cryotherapy is readily demonstrated
on ultrasound, a significant advantage for this approach. Although
generally widely available, the application of cryotherapy for breast
cancer treatment has not been a popular option in research centers
performing minimally invasive therapy. The technology is widely
available, however, and the regulatory requirements are less stringent
than with lasers.
Interstitial hyperthermia is a well-established method for ablating
tissue. First introduced in the late 1980s, a variety of FDA approved
devices are available for the treatment of solid tumors. Interstitial
hyperthermia has been used for the treatment of liver tumors, head
and neck tumors, brain tumors, prostatic enlargement, gynecologic
tumors, and pancreatic carcinoma.1,6,32,39,40,43,50,60,61 Interstitial
hyperthermia was initially applied exclusively with a laser. Other
techniques have emerged including radiofrequency probes, focused
ultrasound, and heated saline. A typical interstitial hyperthermia
treatment would consist of heating for about 10 minutes at a temperature
of about 60(C).
A variety of lasers have been used for interstitial hyperthermia.
Recently, diode lasers have largely replaced NdYAG lasers because
of their small size, portability, use of standard 110 v current,
and lower cost.32 Originally, bare tip fibers were used. There is
debate over the issue of pre-charring the laser tip. Proponents
of pre-charring claim better thermal conduction as a result. Others
claim that light penetrates better if charring does not occur. About
a 1 cm treatment zone can be expected to result from a pre-charred
laser tip when tissue is exposed to 2-3 W of continuous laser power
for about 10 minutes. Recent developments use diffuser tips, extending
the treatment zone to up to 3 cm. Precise temperature control at
the laser tip can be employed to reduce the time of treatment to
about 3 minutes. An advantage of lasers is their small size. A bare
fiber can be inserted through a needle as small as 22 g. The laser
energy may be split into up to four fibers for simultaneous treatment
through multiple needles. Diffuser tip fibers require larger needles
of around 14 g. Laser fibers themselves do not cause MRI artifacts.
The compatibility of lasers with MRI may be helpful as phase sensitive
temperature maps are used for interactive therapy.16
Interstitial hyperthermia may also be delivered with radiofrequency.
FDA approved radiofrequency therapy devices have been developed
for the treatment of colorectal metastases for the liver. These
devices consist of wires that are extended into the tissue which
conduct radiofrequency energy to heat the tissue in a localized
region. Radiofrequency treatment zones are typically up to 3 cm
in diameter, significantly larger than a bare tip laser fiber. Radiofreqency
treatment devices are less expensive than lasers and the regulatory
requirements are not as strict. The disadvantage of radiofrequency
treatment devices is the larger probe size and the use of metal
that produces MRI artifacts.58 Although MRI compatible devices are
made, they are not likely to be compatible with phase sensitive
MRI temperature mapping sequences. The limited use of radiofrequency
probes for breast cancer treatment has been limited to ultrasound
guidance.5
Focused ultrasound has great promise as a minimally invasive treatment
device since it may be applied non-invasively on the skin surface.41
A series of transducers focus the ultrasound energy on a point in
the tissue. This point is rapidly heated to the point of destruction.
Although focused ultrasound is rapid, it does not encompass a large
amount of tissue. In the future, imaging guidance will map a course
for the ultrasound ablation, which will consist of a series of point
ablations that eventually will encompass the entire tumor boundary.
Focused ultrasound is MRI compatible. Machines are being designed
for use inside the bore of the MRI. Focused ultrasound has a disadvantage
in that tissue may move during the course of the therapy, changing
the initial treatment map. Since a needle is not needed in the tissue,
access to the tissue for pathologic confirmation will not be available.
Since pathologic markers are needed for breast cancer, access to
the tumor with a needle is a part of management. If we continue
to require these markers for therapeutic guidance, the non-invasive
advantage of focused ultrasound treatment may not be practically
realized.
How much minimally invasive breast therapy has been performed to
date?
With the societal importance of breast cosmesis and the availability
of current methods, one may ask why more clinical trials are not
open for the validation of minimally invasive therapy of the breast.
Most new therapies are employed first on individuals where there
are few alternatives. For example, tamoxifen was first demonstrated
to be effective in women who failed conventional therapy. Now tamoxifen
is used a first line chemotherapy. Unfortunately, this approach
is not applicable to minimally invasive therapy. The benefits of
minimally invasive therapy can only be achieved in the treatment
of small breast cancers. These cancers are now being treated with
an outstanding prognosis. The ethical dilemma is can we subject
these individuals to a new therapy with unknown effectiveness when
we know the standard treatment has a great potential for curing
the disease? This concern has greatly limited progress on the clinical
implementation of new minimally invasive treatment methods.
Some researchers have addressed this dilemma by testing the methodology
on patients with benign fibroadenomas.41,18 These lesions have negligible
malignant potential, but many women prefer to have them removed.
Fibroadenomas usually present as palpable masses and can produce
pain. Since there is little downside risk, these women can be treated
with the new therapy alone and followed clinically.
Minimally invasive therapy has been used successfully in a small
group of these patients, with excellent results. These findings
illustrate the potential for minimally invasive therapy to improve
cosmesis associated with lumpectomy surgery. Most patients experience
relief of pain and the mass associated with fibroadenomas after
minimally invasive therapy. There is little or no deformity after
the treatment. The treatment of fibroadenomas, however, is not likely
to be a major application of this new therapy. Since fibroadenomas
have little malignant potential and often involute when left alone,
the best treatment option is probably no treatment at all.
Most clinical trials using minimally invasive treatment of breast
cancer have been undertaken in women who subsequently undergo surgery
(22-27). In this situation, the tumor or a portion of the tumor
is treated with the new therapy. After surgical removal, the treated
tumor is examined pathologically for evidence of cell death. The
ability of pathology to accurately determine cell death has been
debated. If the surgery is performed shortly after interstitial
hyperthermia, routine H & E staining may not show evidence of
cell death. Our group prefers to use the proliferating cell nuclear
antigen stain (PCNA), which stains nuclei that have abundant turnover
of DNA. In areas of thermal ablation, there is little PCNA activity,
indicating likely cell death. The PCNA stain works well when the
treatment zone lies within the tumor where the nuclear staining
is apparent. The demonstration of treatment zones in normal tissue,
however, is problematic. If a delay of a few days is achieved between
the minimally invasive therapy and surgery, demonstration of treatment
zones is straightforward. Unfortunately, this delay is impractical
in most American settings. In the United Kingdom, however, such
delays are typical and have facilitated excellent demonstration
of effective treatment.
Ultimately, a clinical trial will be initiated to test minimally
invasive breast cancer treatment. A pilot study that explores the
feasibility of this new therapy in terms of side effects will be
the first step. To determine equivalence of this therapy to surgical
lumpectomy will require a prospective, randomized multicenter trial.
Since the expected five-year recurrence rate will be only a few
percent, large patient numbers will be needed to determine statistical
significance. We have a number of developments that will need to
be standardized before a trial this ambitious can be initiated.
A collaborative effort among industry, academia, and government
will be the most expedient method for launching this effort.
Summary
The success of breast MRI in determining negative predictive value
for breast cancer has made the minimally invasive therapy of breast
cancer a clinical possibility. The challenge for MRI technology
is the development of reliable methods for excluding DCIS as well
as invasive carcinoma. Stereotaxic devices are becoming available
for accurate localization of breast cancers. Greater flexibility
and precision is needed for minimally invasive therapy applications.
Treatment devices are rapidly being developed for other organs that
may benefit breast cancer treatment. Coordinating the delivery of
therapy with the MRI is likely to be a successful approach.
Perhaps the greatest challenge is the development of a clinical
trial that assures patient safety and provides data that would validate
this new treatment as an alternative to traditional surgery. More
work will be needed to provide the tools for this effort. It is
clear that society wants a minimally invasive option as an alternative
to the disfigurement of surgery. The benefits of this new therapeutic
approach are strong incentives that will drive the technological
development in upcoming years.
References
- Amin Z, Donald JJ, Masters A, et al. Hepatic metastases: Interstitial
laser photocoagulation with real-time US monitoring and dynamic
CT evaluation of treatment. Radiology 187:339-347, 1983.
- Bader J, Lippman ME, Swain SM. Preliminary report of the NCI
early breast cancer (BC) study: A prospective randomized comparison
of lumpectomy (L) and radiation (XRT) to mastectomy (M) for Stage
I and II BC (abstract). Int J Radiat Oncol Biol Phys 13 (suppl
1):160, 1987.
- Blichert-Toft M. A Danish randomized trial comparing breast
conservation with mastectomy in mammary carcinoma. Br J Cancer
62 (suppl 12):15, 1990.
- Blichert-Toft M, Brincker H, Andersen JA, et al. A Danish randomized
trial comparing breast-preserving therapy with mastectomy in mammary
carcinoma: Preliminary results. Acta Oncologica 27:671-677, 1988.
- Bohm T, Hilger I, Muller W. Saline-enhanced radiofrequency
ablation of breast tissue: An in vitro feasibility study. Invest
Radiol 35:149- 157, 2000.
- Bown SG. Phototherapy of tumours. World J Surg 7:700-709, 1983.
- Cardwell DM, Harms SE, Lindquist D, et al. Laser directed portable
MRI stereotactic system. Radiology 217P:268, 2000.
- Cross MJ, Harms SE, Cheek JH, et al. New horizons in the diagnosis
and treatment of breast cancer using magnetic resonance imaging.
Am J Surg 166:749-755, 1993.
- Daniel BL, Yen Y-F, Glover GH, et al. Breast disease: dynamic
spiral MR imaging. Radiology 209(2):499-509, 1998.
- Daniel BL, Birdwell RL, Ikeda DM, et al. Breast lesion localization:
freehand, interactive MR image-guided technique. Radiology 207(2):455-463,
1998.
- deSouza NM, Gilderdale DJ, Coutts GA, et al. Magnetic resonance
imaging guided breast biopsy using a frameless stereotactic technique.
Clin Radiol 51:425, 1996.
- Fisher B, Redmond C, Poisson R, et al. Eight-year results of
a randomized clinical trial comparing total mastectomy and lumpectomy
with or without irradiation in the treatment of breast cancer.
New Engl J Med 320:822-828, 1989.
- Fischer U, Vosshenrich R, Bruhn H, et al. Breast biopsy guided
with MR imaging: experience with two stereotaxic systems. Radiology
193(P):267, 1994.
- Fischer U, Vosshenrich R, Keating D, et al. MR-guided biopsy
of suspect breast lesions with a simple stereotaxic add-on-device
for surface coils. Radiology 192(1): 272-273, 1994.
- Ghossein NA, Alpert S, Barba J, et al. Importance of adequate
surgical excision prior to radiotherapy in the local control of
breast cancer in patients treated conservatively. Arch Surg 127:411-415,
1992.
- Graham SJ, Bronskill MJ, Henkelman RM. Time and temperature
dependence of MR parameters during thermal coagulation of ex vivo
rabbit muscle. Magn Res Med 39:198-203, 1998.
- Haga S; Makita M; Shimizu T et al. Histopathological study
of local residual carcinoma after simulated lumpectomy. Surg Today
25:329-33, 1995.
- Hall-Craggs MA. Interventional MRI of the breast: Minimally
invasive therapy. Eur Radiol 10:59, 2000.
- Harms SE, Mumtaz H, Klimberg SV, et al. Magnetic resonance
imaging-directed laser lumpectomy. Breast Diseases: A Year Book
Quarterly 9(4):336-338, 1999.
- Harms SE, Mumtaz H, Hyslop B, et al. RODEO MRI guided laser
ablation of breast cancer. Society of Photo-Optical Instrumentation
Engineers Proceedings 3590:484-489, 1999.
- Harms SE, Flamig DP, Hesley KL, et al. Magnetic resonance imaging
of the breast. Magn Reson Q 8(3):139-155, 1992.
- Harms SE, Flamig DP, Hesley KL, et al. Fat suppressed three-dimensional
MR imaging of the breast. RadioGraphics 13:247-267, 1993.
- Harms SE, Flamig DP, Hesley KL, et al. Breast MRI: rotating
delivery of excitation off-resonance: Clinical experience with
pathologic correlations. Radiology 187:493-501, 1993.
- Harms SE, Flamig DP. MR imaging of the breast: Technical approach
and clinical experience. RadioGraphics 13:905-912, 1993.
- Harries SA, Masters A, Lees WR, et al. Eur J Surg Oncol 19:217,
1993.
- Harries SA, Amin Z, Smith MEF, et al. Interstitial laser photocoagulation
as a treatment for breast cancer. Br J Surg 81:1617-1619, 1994.
- Hatschek T, Fagerberg G, Stal O, et al. Cytometric characterization
and clinical course of breast cancer diagnosed in a population-based
screening program. Cancer 64:1074-1081, 1989.
- Heywang-Kobrunner SH, Schlegel A, Beck R, et al. Contrast-enhanced
MRI of the breast after limited surgery and radiation therapy.
J Comput Assist Tomogr 17(6):891-900, 1993.
- Heywang-Koebrunner SH, Halle MD, Requardt H, et al. Optimal
procedure and coil design for MR imaging-guided transcutaneous
needle localization and biopsy. Radiology 193(P):267, 1994.
- Holland R, Veling SHJ, Mravunac M, et al. Histologic multifocality
of Tis, T1-2 breast carcinomas: Implication for clinical trials
of breast-conserving surgery. Cancer 56:979-90, 1985.
- Hussman K, Renslo R, Phillips JJ, et al. MR mammographic localization.
Radiology 189(3):915-917, 1993.
- Jacques SL, Rastegar S, Motamedi M, et al. Liver photocoagulation
with diode laser (805 nm) vs Nd:YAG laser (1064 nm). Proc SPIE
1646:107-117, 1992.
- Joensuu H, Taikkanen S. Cured of breast cancer. J Clin Oncol
13:62-69, 1995.
- Kuhl CK, Mielcareck P, Klaschik S, et al. Dynamic breast MR
imaging: Are signal intensity time course data useful for differential
diagnosis of enhancing lesions? Radiology 211:101-110, 1999.
- Lagios MD, Westdahy PR, Rose MR. The concept and implications
of multicentricity in breast carcinoma. In Sommers SG, Rosen PP
(eds): Pathology Annual. New York: Appleton-Centur y-Crofts, 1981,
pp 83-102.
- Lee FT Jr, Mahvi DM, Chosy SG, et al. Hepatic cryosurgery with
intraoperative US guidance. Radiology 202:624-632, 1997.
- Leitner SP, Swern AS, Weinberger D, et al. Predictors of recurrence
for patients with small (one centimeter or less) localized breast
cancer (T1a,bN0M0). Cancer 76:2266-2274, 1995.
- Letton AH, Mason EM, Ramshaw BJ. Twenty-year review of a breast
cancer screening project: Ninety-five percent survival of patients
with nonpalpable cancers. Cancer 77:104-106, 1996.
- Masters A, Bown SG. Interstitial laser hyperthermia in tumour
therapy. Ann Chir Gynaecol 79:244-251, 1990.
- Matthewson D, Coleridge-Smith P, O’Sullivan JP, et al.
Biological effects of intrahepatic neodymium:yttrium-aluminum-garnet
laser photocoagulation in rats. Gastroenterology ; 93:550-557,
1987.
- McDannold N, Hynynen K, Wolf D, et al. MRI evaluation of thermal
ablation of tumors with focused ultrasound. JMRI 8:91-100, 1998.
- Mumtaz H, Hall-Craggs MA, Wotherspoon A. Laser therapy for
breast cancer: MR imaging and histopathologic correlation. Radiology
200(3):651-658, 1996.
- Nolsoe CP, Torp-Pedersen S, Burcharth F, et al. Interstitial
hyperthermia of colorectal liver metastases with a US-guided Nd-YAG
laser with a diffuser tip: A pilot clinical study. Radiology 187:333-337,
1993.
- Orel SG, Schnall MD, LiVolsi VA, et al. Suspicious breast lesions:
MR imaging with radiologic-pathologic correlation. Radiology 190:485-493,
1994.
- Orel SG, Schnall MD, Newman RW, et al. MR imaging-guided localization
and biopsy of breast lesions: initial experience. Radiology 193:97-102,
1994.
- Pezner RD, Lipsett JA, Desai K, et al. To boost or not to boost:
decreasing radiation therapy in conservative breast cancer treatment
when “inked” tumor resection margins are pathologically
free of cancer. Int J Radiat Oncol, Biol, Phys 14:873-877, 1988.
- Robinson DS, Parel JM, Denham DB, et al. Interstitial laser
hyperthermia model development for minimally invasive therapy
of breast carcinoma. J Am Coll Surg 186:284-292, 1998.
- Rosen PP, Groshen S, Saigo PE, et al. A long-term follow-up
study of survival in stage 1 (T1N0M0) and stage II (T1 N1M0) breast
carcinoma. J Clin Oncol 7:355-366, 1989.
- Ryoo MC, Kagan AR, Wollin M, et al. Prognostic factors for
recurrence in the conservative management of early breast cancer:
A 25 year follow-up. Int J Radit Oncol Biol Phys 17:719-725, 1989.
- Sarrazin D, Le MG, Arriagada R, et al. Ten-year results of
a randomized trial comparing a conservative treatment to mastectomy
in early breast cancer. Radiother Oncol 14:177-184, 1989.
- Schatz SW, Bown SG, Wyman DR, et al. Low power interstitial
Nd-YAG laser photocoagulation in normal rabbit brain. Lasers Med
Sci 7:433-439, 1992.
- Schmidt-Ullrich R, Wazer DE, Tercilla O, et al. Tumor margin
assessment as a guide to optimal conservation surgery and irradiation
in early stage breast carcinoma. Int J Radiat Oncol Biol Phys
17:733-738, 1989.
- Schnall MD, Orel SG, Connick TJ. MR guided biopsy of the breast.
MRI Clin No Am 4:585-590, 1984.
- Schnitt SJ, Abner A, Gelman R, et al. The relationship between
microscopic margins of resection and the risk of local recurrence
in patients with breast cancer treated with breast-conserving
surgery and radiation therapy. Cancer 74:1746-1751, 1994.
- Schwartz GF, Patchesfsky AS, Feig SA, et al. Multicentricity
of nonpalpable breast cancer. Cancer 45:2913-2916, 1980.
- Seidman H, Gelb SK, Silvergerg E, et al. Survival experience
in the breast cancer detection demonstration project end results.
CA Cancer J Clin 5: 258-290, 1987.
- Soderstrom CE, Harms SE, Copit DS, et al. 3D RODEO breast MRI
of lesions containing ductal carcinoma in situ. Radiology 201:427-
432, 1996.
- Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases:
Percutaneous radio-frequency ablation with cooled-tip electrodes.
Radiology 205:367-373, 1997.
- Spivak B, Khanna MM, Tafra L, et al. Margin status and local
recurrence after breast-conserving surgery. Arch Surg 129:952-957,
1994.
- Steger AC, Lees WR, Walmsley K, et al. Interstitial laser hyperthermia:
A new approach to local destruction of tumors. Br Med J 299:362-365,
1989.
- Storm FK, Sliberman AW, Ramming KR, et al. Clinical thermochemotherapy:
A controlled trial in advanced cancer patients. Cancer 53:863-868,
1984.
- Veronesi U, Banfi A, Del Vecchio M, et al. Comparison of Halsted
mastectomy with quadrantectomy, axillary dissection, and radiotherapy
in early breast cancer: Long-term results. Eur J Cancer Clin Oncol
22:1085-1089, 1986.
| Cryosurgery: Does it Have a Role in the
Breast? Susan M. Love, M.D. MBA, Adjunct Professor of Surgery
UCLA, Pacific Palisades, CA |
 |
Surgical excision has been the gold standard for dealing with benign
and malignant breast lesions for decades. The argument for surgical
removal of a lesion rested on the need to determine pathological
information as well as to measure markers. Removing the lesion has
the added benefit of local control. The increasing use of large
core needle biopsies under ultrasound or stereotactic guidance has
opened the way to a reevaluation of the need to remove breast lesions.
Pathology and markers can easily be measured in large core biopsies.
This leaves us with the question as to whether there are other ways
to ablate tumors and achieve local control.
Since the effectiveness of cryosurgery for ablation of for skin,
hepatic, ocular lesions and prostate cancers had been demonstrated,
it is reasonable to evaluate its use in the breast. We established
a model in C3H mice of 1 cm subcutaneous breast cancers. After anesthetizing
the mice we employed a 1 mm cryolite probe (Cryomedical Sciences,
Rockville MD) to freeze the tumor while monitoring the temperature
in the center of the tumor, margin of the tumor and centrally in
the mouse. First we evaluated varying freeze thaw cycles, tumor
temperatures, margin temperatures and time to thaw. Animals who
had their tumors frozen to -40o for three freeze thaw cycles demonstrated
a loss of tumor mass and residual wet or dry necrosis with disappearance
of the tumor mass between 12-20 days. In this initial phase thirteen
animals had complete disappearance of tumor and four animals had
recurrence of tumor. In this pilot study local ablation of breast
tumors with cryosurgery was a feasible alternative to surgery in
mice.
Cryosurgery is particularly well suited for use in the breast for
a number of reasons. First of all, the ice ball that is created
is extremely well visualized on ultrasound due to its highly echo-reflective
properties. This allows for very accurate monitoring of ice ball
growth, a high degree of procedural control, and a tailoring of
the therapy directly to the pathology. Sterile normal saline or
local anesthetic can be repeatedly injected between the skin and
forming ice ball and serves as a protective barrier. The ultra-cold
temperatures created by modern cryoablation systems are anesthetic
in nature, thus allowing adequate treatment using local anesthesia
without sedation.
In a study sponsored by Sanarus a 2.4-mm cryoprobe was used under
two different protocols to evaluate its effectiveness in both benign
and malignant disease. Fifty-seven biopsy-proven fibroadenomas were
treated in 46 patients. Early in the study, 10 patients were treated
in an ambulatory surgery setting with moderate intravenous sedation
and local anesthesia. The remaining patients were treated in an
office setting. The first 18 of these patients treated in the office
received local anesthesia with 100 mcg of I.V. Fentanyl. All subsequent
office-based treatments were delivered using local anesthesia only.
The first 22 fibroadenomas in 19 patients were treated with a 10-minute
freeze followed by a 10-minute thaw and another 10-minute freeze.
No cryo-related findings (e.g. calcifications or scarring) were
noted on these patient’s mammograms at 6- or 12-months post
treatment. The subsequent fibroadenomas were treated with an algorithm
based on tumor size that entailed decreased treatment times and
reduced gas flow in order to more closely tailor the therapy to
the tumor. This algorithm provides a total treatment time (double
freeze and thaw) of 18 minutes for a 2-cm fibroadenoma. Mean pre-treatment
maximum tumor diameter by ultrasound for all tumors was 1.9 + 0.8
cm. In 25 tumors that have reached 12-month follow up, average tumor
volume reduction was 90%. No significant complications were noted.
One mastitis and two hematomas responded to oral antibiotics and
time, respectively. Cosmetic results have been outstanding.
A pilot study is ongoing to evaluate the use of cryoablation in
core biopsy proven breast cancers that are to be resected one to
three weeks later. Of the first eleven patients treated in this
series, four were treated in ambulatory surgery under local anesthesia
with intravenous sedation. The others received only local anesthesia
in the office. Tumors had a mean maximum diameter of 13-mm. One
case had a double 8-minute freeze with an intervening 8-minute thaw.
The others were double ten-minute freezes. All but one patient had
their tumors completely destroyed by the cryoablation procedure.
This patient had an area of DCIS at the margin.
Cryoablation is an excellent modality for the treatment of breast
fibroadenomas today. It use in malignant tumors could include cryolumpectomy
as well as cryoablation to achieve local control. Can interstitial
ablation be used to destroy breast tumors locally through tiny skin
incisions? The answer is clearly yes. Radiofrequency, laser, high
frequency ultrasound, and cryoablation have all been demonstrated
to successfully do so. What remains to be elucidated is the appropriate
clinical application of these technologies. And the questions will
be not only are these ablative techniques as good as surgery but
could they be better? Could there be immunological benefits to any
of these techniques? Could the ablation of a tumor either immediately
prior to or in place of its surgical excision have an impact on
the spill of tumor cells in the blood compared to lumpectomy? Would
such an impact have clinical relevance? Of obvious concern is the
issue of margin evaluation in cases where a breast cancer is not
going to be removed. But improvements in imaging and adjuvant therapies
that may render this question less important. Less obvious in the
definition of “minimally invasive” are such items as the
ability of an interstitial ablation technique to demonstrate superior
cosmetic results or to be performed using strictly local anesthesia
in an office environment that will be more comfortable for the patient.
| Brachytherapy to the Tumor Bed Alone Versus
Whole Breast Irradiation Frank A. Vicini, M.D., Clinical Assoc.
Professor, Director Radiation Oncology, William Beaumont Hospital,
Royal Oak, MI |
 |
Introduction: The combination of lumpectomy followed by radiation
therapy (RT), referred to as breast conserving therapy (BCT) is
now widely accepted as an equivalent treatment option for most women
with clinical stage I/II invasive breast cancer. One question that
has remained unanswered, however is whether or not the entire breast
needs to be treated, or only a more limited volume of tissue surrounding
the tumor bed. Traditionally, patients treated with BCT have been
irradiated to the entire breast. However, most of the potential
long-term complications and time constraints of BCT result from
either whole-breast or nodal irradiation. In addition, giving radiotherapy
to the entire breast essentially precludes giving further RT in
the event that a patient develops a new primary tumor.
In recognition these problems, the option of partial-breast irradiation
(PBI) has been explored. This concept lends itself to much shorter
treatment schemes than whole breast irradiation, as the toxicities
to the breast and surrounding tissues should be lessened by treating
only a portion of the breast. In addition, if a significantly shortened
treatment scheme could be shown to produce equivalent outcome to
traditional six weeks of whole breast irradiation, it would substantially
improve the quality of life of patients and allow easier integration
of radiotherapy with chemotherapy. Just as important, a logistically
simpler and quicker treatment could potentially increase the breast
conservation option to more women and reduce the costs of post-lumpectomy
radiation. To this end, several groups began to explore accelerated
treatment schemes in the 80’s and 90’s. Both external
beam radiation therapy (EBRT) and brachytherapy were employed(1-14).
The success of these early phase I/I and III studies has been inconsistent
and will be reviewed as well as the outcome of PBI using brachytherapy
alone at William Beaumont Hospital.
Materials and Methods: From 1/93 through 1/02, 197 cases of early
stage breast cancer were managed with lumpectomy followed by RT
restricted to the tumor bed using an interstitial implant (120 cases
using a low dose rate implant (50 Gy over 96 hours) and 77 cases
using a high dose rate implant (32 Gy in 8 fractions over 4 days
or 34 Gy in 10 fractions over 5 days). Locoregional control, distant
metastases (DM), disease free survival (DFS), overall survival (OS),
and cause-specific survival (CSS) were calculated.
In order to estimate the effects of selection bias and short follow-up
on treatment results, each brachytherapy patient was matched with
one EBRT patient derived from a reference group of 1388 patients
treated with standard BCT at the same institution. Patients were
matched for age, tumor size, histology, margins of excision, absence
of extensive intraductal component (EIC), nodal positivity, estrogen
receptor status, the use of adjuvant tamoxifen, and the use of adjuvant
chemotherapy. Follow-up for EBRT patients was truncated at the same
time interval as each matched brachytherapy patient. Median follow-up
for the EBRT and brachytherapy groups was 50 months and 50 months,
respectively.
Results: Three local and one regional failures were detected in
patients treated with brachytherapy alone (5-yr actuarial rates
of 1.9% and 1.3%, respectively. Three patients failed distantly.
No adverse sequelae were noted and cosmetic results were good/excellent
in > 90% of patients. No statistically significant differences
were noted in the 5-year actuarial rates of ipsilateral failure,
locoregional failure or DM between EBRT or brachytherapy patients.
Conclusions: Accelerated treatment of breast cancer using an interstitial
implant to deliver RT to the tumor bed alone in 4-5 days appears
to produce equivalent 5-year results compared to conventional EBRT.
Extended follow-up will be required to determine the long-term efficacy
of this treatment approach and the potential advantages/disadvantages
of accelerated treatment.
Reference List
- Kuske, R. R., Bolton, J. S., and Hanson, W. RTOG 95-17: A phase
I/II trial to evaluate brachytherapy as the sole method of radiation
therapy for stage I and II breast carcinoma. 1-34. 1998.
- King TA, Bolton JS, Kuske RR, Fuhrman GM, Scroggins TG, Jiang
XZ. Long-term results of wide-field brachytherapy as the sole
method of radiation therapy after segmental mastectomy for T(is,1,2)
breast cancer. Am.J Surg 2000;180:299-304.
- Perera F, Engel J, Holliday R, Scott L, Girotti M, Girvan D
et al. Local resection and brachytherapy confined to the lumpectomy
site for early breast cancer: a pilot study. J Surg Oncol 1997;65:263-7.
- Polgar, C., Fodor, J., Orosz, Z., Major, T., Takacsi-Nagy,
Z., Mangel, L. C., Somogyi, A., Sulyok, Z., Toth, J., and Nemeth,
G. Sole high-dose- rate brachytherapy of the tumor bed after conservative
surgery for T1 breast cancer: 4 year results of a phase I-II study
and initial findings of a randomized phase III trial. 2001.
- Vicini FA, Baglan KL, Kestin LL, Mitchell C, Chen PY, Frazier
RC et al. Accelerated treatment of breast cancer. J Clin.Oncol
2001;19:1993- 2001.
- Fentiman IS, Poole C, Tong D, Winter PJ, Mayles HM, Turner
P et al. Iridium implant treatment without external radiotherapy
for operable breast cancer: a pilot study. Eur.J Cancer 1991;27:447-50.
- Fentiman IS, Poole C, Tong D, Winter PJ, Gregory WM, Mayles
HM et al. Inadequacy of iridium implant as sole radiation treatment
for operable breast cancer. Eur.J Cancer 1996;32A:608-11.
- Cionini, L. Pacini P. Marzano S. et al. Exclusive brachytherapy
after conservative surgery in cancer of the breast. Lyon Chir
89, 128. 1993.
- Clarke DHVFAJHea. High dose rate brachytherapy for breast cancer.
In: Nag S. High dose rate brachytherapy: A textbook, First ed.
Armonk, N.Y.: Futura Publishing, 1994:321-9.
- Ribeiro GG, Magee B, Swindell R, Harris M, Banerjee SS. The
Christie Hospital breast conservation trial: an update at 8 years
from inception. Clin.Oncol (R.Coll.Radiol) 1993;5:278-83.
- Ribeiro GG, Dunn G, Swindell R, Harris M, Banerjee SS. Conservation
of the breast using two different radiotherapy techniques: interim
report of a clinical trial. Clin.Oncol (R.Coll.Radiol) 1990;2:27-34.
- Magee B, Swindell R, Harris M, Banerjee SS. Prognostic factors
for breast recurrence after conservative breast surgery and radiotherapy:
results from a randomised trial. Radiother.Oncol 1996;39:223-7.
- Wazer DE, Lowther D, Boyle T, Ulin K, Neuschatz A, Ruthazer
R et al. Clinically evident fat necrosis in women treated with
high-dose-rate brachytherapy alone for early-stage breast cancer.
Int J Radiat Oncol Biol Phys 2001;50:107-11.
- Krishnan L, Jewell WR, Tawfik OW, Krishnan EC. Breast conservation
therapy with tumor bed irradiation alone in a selected group of
patients with stage i breast cancer. Breast J 2001;7:91-6.
| Intraoperative Full-Dose Radiotherapy in
Limited-Stage Breast Cancer Conservatively Treated Umberto Veronesi,
M.D., Scientific Director, European Institute of Oncology, Milan,
Italy |
 |
The development of Intraoperative Radiotherapy was based on the
evidence that local recurrences after breast conserving surgery
occur mostly in the quadrant harbouring primary carcinoma. The main
objective of postoperative radiotherapy appears therefore to be
the sterilization of residual cancer cells in the operative area
while irradiation of the whole breast may be avoided. We have developed
a new technique of intraoperative radiotherapy of a breast quadrant
after the removal of the primary carcinoma. A mobile linear accelerator
with a robotic arm is utilized delivering electron beams able to
produce energies from 3 to 9 MeV. Through a perspex applicator the
radiation is delivered directly to the mammary gland and to spare
the skin from the radiation the skin margins are stretched out of
the radiation field. To protect the thoracic wall an aluminium-lead
disc is placed between the gland and the pectoralis muscle.
Different dose-levels were tested from 10 to 21 Gy without important
side effects. We estimated that a single fraction of 21 Gy is equivalent
to 60 Gy delivered in 30 fractions at 2 Gy/fraction.
Seventeen patients received a dose of IORT of 10 to 15 Gy as an
anticipated boost to external radiotherapy, while 86 patients received
a dose of 17-19-21 Gy intraoperatively as a complete treatment.
The follow-up time of the 101 patients varies from 15 to 30 months
(mean follow-up time 20 months).
The IORT treatment was very well accepted by all patients, either
due to the rapidity of the radiation course in case of IORT as a
whole treatment or to the shortening of the subsequent external
radiotherapy in case of IORT as an anticipated boost. We believe
that single dose intraoperative radiotherapy after breast resection
for small mammary carcinomas may be an excellent alternative to
the traditional postoperative radiotherapy.
| Accelerated Treatment with Increased Fractionation
for Breast Irradiation Tim Whelan, B.M., B.Ch., M.Sc., Associate
Professor, Dept. of Medicine and Director, Supportive Cancer
Care Research Unit, McMaster University, Ontario, Canada |
 |
A number of randomization trials have demonstrated that breast
irradiation following lumpectomy reduces the risk of recurrence
of cancer in the breast and prevents the need for mastectomy. Breast
irradiation is usually given daily for five to six weeks. However,
the optimal fractionation for breast irradiation is unclear. Retrospective
studies have suggested that more rapid fractionation schedules for
breast irradiation may be as effective. A randomized controlled
trial was performed in Canada to determine whether a three week
course of radiation (42.5 Gy in 16 fractions administered over 22
days) was as effective a five course (50 Gy in 25 fractions administered
over 35 days) in women with node negative breast cancer with clear
resection margins following lumpectomy. The primary outcome was
local recurrence in the treated breast. An important secondary outcome
was breast cosmesis as measure of late morbidity for radiation therapy.
Between April of 1993 and September of 1996, 1,234 women were randomized.
Median follow-up is now more than five years. Rates of local recurrence
free survival were similar in both treatment arms. Cosmetic outcome
assessed at three and five years post randomization was also similar
between the two treatment groups. The results of this trial are
consistent with earlier studies of hypofractionation and support
that the shorter three-week schedule of radiation is as an acceptable
alternative to the longer five-week schedule. Such a schedule is
attractive because it is more convenient for patients and less resource
intensive.
| How to get around involved margins an anatomical
challenge Werner P. Audretsch, M.D., Chief Dept. of Breast Surgery
Metropolitan Hospital, Dusseldorf, Germany |
 |
Classification
INVOLVED MARGINS are classified by microscopic examination as R1
or by intraoperative examination as R2 resection. Untreated involved
margins are a marker and a precursor of a limited outcome.
Problem
In studies of our own, we have investigated the influence of biological
and anatomical factors on local recurrence and the influence on
prognosis in 2875 patients treated with total or partial mastectomy.
Both histological-anatomical characteristics and tumour biological
factors influence the local risk to an equal degree. It is striking
that an early local recurrence leads to an extremely bad prognosis.
This is especially the case following partial mastectomy. In the
analysis of multiple variance, the factor margin is highly significant
as an independent predictor (P=0.0002) for loco-regional early recurrence.
This is followed by the biological factor (P=0.0039) progesterone
receptor and by tumour size (P=0.0382).
Diagnosis
The assessment is based on a three-dimensional reconstruction of
the extension of the lesion in the tumor specimen by the pathologist.
Type of involvement:
- Surrounding matrix
- Deep margins
- Overlaying skin
- Margin’s status together with local control are the significant
determinants of surgical quality.
Etiology
- Young patients (<35 y, EORTC 22881)
- Non palpable lesion
- Non palpable diameter of the lesion extending the palpable mass
- Partial occult lesion larger than expected from standard imaging
- Gaps between foci
- Extended intraductal component
- Tumors in the border line of the breast close to the chest wall
- Lymph vessel invasion
Prognostic value
Results from a worldwide overview (Richard Peto, NIH Consensus,
November 2000) suggest that a quarter of isolated local recurrences
will result in death from breast cancer, deaths that would not otherwise
have happened. Avoiding 20 local recurrences will lead to the avoidance
of 5 breast cancer deaths in 15 years.
The EORTC-Study 22881 has made clear the significance of free margins
for prognosis, especially in young patients below the age of 35
years.
Looked at from this point of view, the creation of free margins
in connection with a cosmetic result is a decisive factor in BCT
and in cases of mastectomy reconstruction.
Treatment
Cascade of decisions:
- Re-excision; but “first bite is the best” (Silverstein)
- Radiotherapy; but “bad surgery can not always be solved
by good radiotherapy” (Batelink)
- Systemic chemotherapy; has a local effect (NSABP B6, NSABP-B18)
- Systemic hormontherapy; has a local effect (Neo-adjuvant anastrozole
trial)
- Skin sparing mastectomy or total mastectomy following breast
conserving surgery
- Combined radiotherapy and chemotherapy for partial mastectomy
with R1 resection (in cases of >3 node positive primaries);
results from our case control study: only 22% ypT+, residual tumor
i.e. 78% ypT0, complete pathologic response after salvage mastectomy.
- Surveillance including mammogram, ultrasound, MRI
- Salvage mastectomy in case of uncertain local symptoms
The NSABP-B18-Study was able to prove the equal value of a preoperative
and adjuvant chemotherapy.
Prevention
- Ultrasound needle framing of the mass
- MRI for breast conserving surgery in young patients
- Widening of excision without distorsion
Oncological and reconstructive operative procedures for the prevention
and the solution of this conflict of aims can be summarised under
the term oncoplastic surgery or coupled operations for partial and
total mastectomies.
| Skin Sparing Mastectomies Rache M. Simmons,
MD FACS, Associate Professor of Surgery, Strang Weill-Cornell
Breast Center, New York Presbyterian Hospital Weill Medical
College of Cornell University, New York, NY |
 |
Skin Sparing Mastectomy and Immediate Breast Reconstruction
- Fortunately, most women diagnosed with breast cancer can be
treated with breast conserving surgery.
- With those women who require or desire mastectomy, consideration
should be given to optimize the cosmetic result with skin sparing
mastectomy and immediate breast reconstruction
Advantages of Immediate Breast Reconstruction with Skin Sparing
Mastectomy
- Improve body image and decrease psychological trauma of a breast
cancer diagnosis
- Medically beneficial to avoid additional episode of anesthesia
and recovery
- More cost effective
- Many patients will not pursue second operation for delayed reconstruction
Skin Sparing Mastectomy (SSM) Resection of nipple/areolar complex
Resection of existing biopsy scar Removal of entire breast parenchyma
+/- Axillary dissection Limited skin incision
SSM Incisions
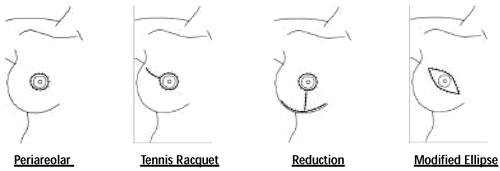
Additional Incision Types with SSM • Periareolar with medial
and lateral extensions 1 • Separate incision in the axilla
to facilitate axillary dissection2,3
1 Slavin, et al, PRS, 102, 1998
2 Hildalgo, PRS, 102, 1998
3 Toth, PRS, 104, 1999
Aesthetic Advantages of SSM
- Smaller incisions
- Minimal skin resected in procedure
– Skin envelope retained
- Improved symmetry with contralateral breast
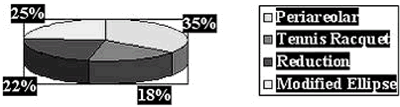
Simmons, Ann Surg Onc, 6, 1999
Is Axillary Dissection Possible with SSM?
- Access through any of SSM incisions
- No decreased ability to remove axillary contents or perform
sentinel node biopsy
- Average number of lymph nodes removed with SSM 18.0 and with
NSSM 19.0
- Are There Increased Local Complications with SSM?
- Technically more challenging than NSSM
- More limited exposure through smaller incisions
Higher potential for flap injury or loss??
Native Skin Flap Epidermolysis/Skin Loss•
Breast Cancer Stage with SSM
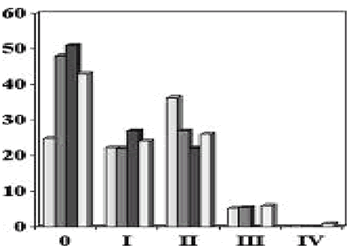
Simmons, Ann Surg Onc, 6, 1999
Toth, PRS, 104, 1998
Slavin, PRS, 102, 1998
Carlson, Ann of Surg, 225, 1997
Local Recurrence Rate with SSM Simmons, SSO Abstract 2002 Carlson,
Ann Surg, 225, 1997 Newman, Ann Surg Onc, 5, 1998 Distant Recurrence
Rate with SSM SSM 2.9% (3/103) NSSM 1.5% (2/134) P=NS Simmons, SSO
Abstract 2002
Methods of Immediate Reconstruction
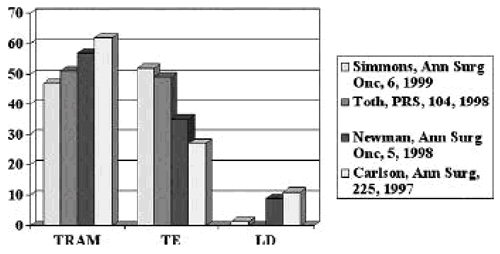
Skin Sparing Mastectomy Conclusions Compared to NSSM:
- SSM with immediate reconstruction offers superior aesthetic
results
- SSM has comparable local and distant recurrence rates
- No higher incidence of skin flap loss
SSM should be considered:
- DCIS, T1 and T2 carcinoma, T3 without skin involvement
- With or without sentinel node biopsy or axillary dissection
- In conjunction with all types of immediate reconstruction
- No contraindication to adjuvant chemotherapy or radiation therapy
SSM not indicated:
- Skin involvement or Inflammatory breast carcinoma
Areolar-Sparing Mastectomy
- Historically mastectomy has included resection of the nipple/areolar
complex
- Assumed increased recurrence risk should the complex not be
removed
- Studies have shown that malignant involvement of an areola/nipple
to be present in 5%-43% of mastectomy specimens
None of the studies separate the respective involvement of the
nipple versus the areola.
- Retrospective analysis 217 mastectomy specimens for nipple or
areola involvement separately
- Nipple involvement 6% - 27% of patients
- Areolar involvement in <1% of patients – 0% DCIS –
0% invasive tumors <5cm – 0% tumors away from retro-areolar
area
Simmons, Annals of Surgical Oncology, 2002
The Next Step: Areolar-Sparing Mastectomy
- Selected patients will be offered to spare the areola with removal
of only the nipple: – DCIS – Prophylactic mastectomies
– ?distal small invasive cancers
| Converting a complicated outcome into a
good reconstruction Werner P. Audretsch, M.D., Chief Dept. of
Breast Surgery, Metropolitan Hospital, Dusseldorf, Germany |
 |
Classification
COMPLICATED RESULTS after breast surgery are related to the diagnosis,
volume of resection, location of the lesion, the type an method
of the surgical technique, the volume of the breast and the experience
of the surgeon and his surgical skill. Other risk factors are the
physical conditions of the patient, age, weight, diabetes and smoking.
Problem
It remains difficult to provide precise numbers of the extension
and specific incidence rates on the diverging data in the literature.
In addition the spectrum of surgical interventions of the breast
was enlarged during the last decades and has enabled the occurrence
of new problems. The indication for breast conserving surgery have
increased after neo-adjuvant treatment. The aesthetic results after
true quadrantectomy need revision in 20% of cases and are limited
particularly with small breasts in 90%. Therefore, the factors of
unfavourable relative size and unfavourable site belong to the relative
contraindications of breast conservative treatment.The results of
the EORTC-10801- Study show that there is basically no oncological
contraindication for BCT because of tumor size or involvement of
the lymph nodes alone. There is a growing number of different implant
and autologous procedures of breast reconstruction. In the same
way more functional techniques of breast cancer surgery and for
for the axilla i.e. sentinel lymphe node biopsy are gaining importance.
A specific aspect of breast surgery is the fact that a reduced cosmetic
outcome counts for a complication.
Diagnosis
The assessment is based on esthetic principles of the breast:
Etiology
- wrong technique (implant, autologous, combination)
- wrong patient (indication, sequence to other treatment)
- wrong doctor (experience, specific training, lack of interdisciplinary
approach)
Due to the implicit oncological point of surgery for breast cancer
, plastic and reconstructive surgery requires considerations of
treatment sequence for CHT and radiotherapy. However, an immediate
reconstruction cannot be performed without a meticulous preoperative
evaluation of the cancer status and an upfront planning during the
treatment conference.
Treatment
Autologous local tissue or transferred tissue can be used to cover
defects or to convert a failed implant reconstruction. Local tissue
is used, for example, in cases of defect shrinking achieved through
different volume reduction techniques i.e. reduction mammoplasty,
and mastopexy techniques involving different defect-adapted skin
patterns such as the standard key-hole pattern, the modified “B”
technique developed by Regnault and applied to central and inner-lower
pole lesions, the oblique pattern, the “purse-string”
pattern , the inverted “T”-pattern for low-pole lesions,
and the inverted Rubin pattern preferred for other kinds of defect
location in large or pendulous breasts. A thoraco-epigastric flap
(TEF) or thoraco-dorsal flap (TDF) may be recommendable for reconfiguring
the infero-medial and infero-lateral aspect of the breast. Local
autologous tissue is taken from the area adjacent to the tumor bed
or the chest wall and should be radiated after surgery in cases
of breast-conserving treatment.
Distant and transferred autologous tissue is used in partial or
total mastectomy reconstruction on behalf of a myocutaneous latissimus
dorsi flap (LAT) for lesions in the unfavourable borderline of the
breast and for relative size problems or together with an implant.
This kind of tissue is reliable as long as the thoraco-dorsal pedical
and/or the serratus branch remains intact. Because of its twofold
(either simultaneous or successive) availability and its paired
occurrence, in which it resembles the breast, the most important
and best suited “work horse” for the coupled surgical
approach as well as for volume (i.e. “mini-flap”) and
skin replacement is the myocutaneous latissimus dorsi island flap.
The evolution of the latiss from the safest tool in delayed post
radiation repair surgery or deformities to the mainstay in cases
of reconstruction after partial or total mastectomy with regard
to new treatment protocols represents a breakthrough in the field
of breast cancer surgery. The decision between partial and total
mastectomy operability is brought about solely by the distribution
of the tumor in the breast. The myocutaneous rectus flap (TRAM)
has become the state of the art in total mastectomy reconstruction.
Looked at from this point of view, coupled reconstructive surgery
is able to facilitate mastectomy operability as well or to support
a delayed salvage surgery.
Transferred tissue is generally considered healthy and free from
tumor cells. Generally, radiotherapy is not indicated from an oncological
point of view and therefore be applied prior to surgery. In individual
cases radiotherapy can also take place after latissimus partial
or total mastectomy reconstruction but not recommended after TRAM-flap
reconstruction. A simpler reconfiguration can follow by means of
mirror biopsy. The preference of bilateral surgery for Q.U.A.R.T.
and of the recentralization of the N.A.-complex was based on the
aim of restoring symmetry after quadrantectomy or, in cases of asymmetry,
of favouring the non-affected breast.In most of the cases the basis
of the breast is narrowed. This results in a decentralization and,
most frequently, in a lateralization of the N.A.-complex. Consequently,
recentralization should be performed by means of a peri-areolar
concentric mastopexy, i.e. the “purse string” technique.
A response-dependent indication for larger plastic intervention
with the aim of creating a tumor cell free operative zone is desirable.
Prevention
In view of the fact that adjuvant to oncological surgery, radiotherapy,
and chemotherapy, plastic and reconstructive surgery becomes increasingly
integrated into the comprehensive management of breast cancer, it
goes without saying that breast reconstruction in general and above
all coupled procedures require an even more sophisticated and detailed
pre-treatment planning and sequencing. Moreover, sophisticated integration
of plastic surgery together with modern implants, autologous tissue
and the approach of onco-plastic surgery may resource costs and
help to save money because of the aim of predictable or a one-step
procedure, fewer re-excision problems, non-delayed reconstructions,
avoidance of secondary surgery and complications after partial and
total mastectomy, and involves well indicated or fewer contralateral
procedures as in most cases it is a natural breast that is reconstructed.
| Sequence of endocrine therapy for metastatic
breast cancer in pre- and postmenopausal women C. Kent Osborne,
M.D., Professor of Medicine & Cell Biology, Baylor College
of Medicine, Houston, TX |
 |
Endocrine therapy can be classified according to its mechanism
of action. These include therapies designed to reduce the estrogen
level, selective estrogen receptor modulators such as tamoxifen
that have partial agonist activity, ER downregulators and pure antiestrogens
such as Faslodex, and the pharmacologic administration of estrogens,
androgens, and progestins. In postmenopausal women there is now
considerable evidence demonstrating that aromatase inhibitors are
superior to tamoxifen as first line therapy for metastatic breast
cancer. Both Faslodex and the aromatase inhibitors are effective
in patients resistant to tamoxifen, and Faslodex has been shown
to be at least as effective as Arimidex in this setting. Aromatase
inhibitors may be particularly more effective than tamoxifen in
tumors that overexpress the HER-2 oncogene. In premenopausal patients
ovarian ablation by surgery or through LHRH agonists remains effective
treatment, as does the antiestrogen tamoxifen. Some data suggest
that the combination of ovarian ablation plus tamoxifen is superior
to either agent alone. Aromatase inhibitors are not effective in
premenopausal women with functioning ovaries, but they can be considered
in patients who have had prior ovarian ablation. Faslodex also has
not been studied in premenopausal patients, but it can also be considered
in those who have lost ovarian function. The optimal sequence of
endocrine therapy has not been carefully defined, especially considering
the new agents now available. One strategy in premenopausal patients
would be to be to consider ovarian ablation with or without tamoxifen
as initial therapy with aromatase inhibitors or Faslodex as second
and third line treatments. In postmenopausal women, aromatase inhibitors
or even still tamoxifen can be considered initial treatment with
the alternative or Faslodex reserved for secondary and tertiary
treatment. Pharmacologic treatment with high doses of estrogens,
androgens, or progestins is reserved for fourth and fif th line
treatment. Using these sequences of endocrine therapy many patients
can be controlled for years before requiring more aggressive and
toxic cytotoxic chemotherapy.
| Choice of Cytotoxic Regimen: Single vs Combination,
Sequential vs Combination, Dose-Density vs High Dose Clifford
Hudis, M.D., Chief, Medical Oncology of Breast Center, Memorial
Sloan-Kettering Cancer Center, New York City, NY |
 |
The meta-analyses performed by the Early Breast Cancer Trialists’
Collaborative Group clearly demonstrate that combination chemotherapy
in the adjuvant setting significantly reduces the risks of relapse
and, to a lesser extent, death. The three drug combination comprising
CMF given for about 6 months represents a gold standard but it is
likely that other regimens will be found to be consistently superior.
Clinical research in recent years has focused on the use of dose-escalated
therapy and the role of new active drugs, such as anthracylines,
taxanes, and others. Despite pre-clinical models suggesting significant
benefits to dose-escalation and intensity as well as a large number
of promising phase II studies, a clear and consistent benefit for
higher dose therapy has not been seen, especially when considering
dose levels significantly above standard. As a result high dose
therapy appropriately remains investigational. Alternative means
of increasing chemotherapy effect include the use of dense treatment
plans designed to overcome resistance by increasing the exposure
of tumor cells to drug and the incorporation of new active agents.
Dose-Escalation
Laboratory evidence for a steep dose to response relationship, in
particular for alkylating agents, initially led to numerous feasibility
and pilot trials testing the use of very high dose treatments in
patients. To support patients through these treatments autologous
stem cells, collected from the marrow or, more recently, peripherally,
were harvested and re-infused following the high dose treatment.
Because kinetic models of tumor growth and chemotherapy response
suggested that the greatest likelihood for cure would be in the
minimal tumor burden situation, patients with high risk early stage
disease were considered an ideal testing ground for this approach.
Promising non-randomized results allowed high dose adjuvant chemotherapy
to become very popular even before prospective randomized data was
available. Now, however, there have been three such studies published
in manuscript form and several more as abstracts only and, while
one can not rule out a benefit for this approach, the available
results do not demonstrate a significant benefit. Additional disappointment
was negative seen in the results of studies testing dose escalation
for cyclophosphamide above 600 mg/m2 from the NSABP trials B22 and
B25 and another testing doxorubicin dose-escalation above 60 mg/m2
in the recent CALGB trial (9344). For all of these reasons, clinicians
should remain cautious regarding the use of maximally dose-escalated
therapy outside of properly conducted prospectively randomized trials
unless and until there is clearer evidence of their benefit.
Dose Dense Chemotherapy Regimens
Dose-intensity quantifies the total amount of drug administered
over a specified period of time and expresses it in terms of milligrams
per meter-squared per week. Increases in dose-intensity are therefore
possible not only by increasing dose size (dose-escalation) but
also by decreasing the interval between treatments (shortening the
time period). The latter method for dose-intensification, first
proposed by Norton and Simon, can be labeled “dose-dense”
treatment to distinguish it from all other dose-intensification
schemas. A trial conducted in Milan and enrolling women with 4 or
more involved nodes was one of the best tests of dose-dense therapy.
Here treatment consisted of alternating (less dose-dense) versus
a sequential (more dose-dense) regimens using single agent doxorubicin
(A) and CMF and over 33 weeks of treatment every patient on the
study received 4 doses of doxorubicin and 8 of CMF. With 10 years
of follow-up the sequential plan consisting of 4 cycles of doxorubicin
followed by 8 of CMF was associated with a significant improvement
in disease-free and overall survival compared to the alternating
one. Based on these results plus those of several promising pilot
trials the North American Intergroup conducted several trials where
dose-density was the critical question. One study compared concurrent
AC versus sequential (more dose-dense) administration of the same
cumulative doses of these drugs, another compares every other week
chemotherapy with every third week treatment, and yet another compares
a dose-dense regimen versus a “conventional” high dose
therapy approach consisting of AC followed by a single cycle of
high dose therapy. Results from these randomized studies are awaited.
| Taxanes: Update on use in adjuvant and metastatic
setting I. Craig Henderson, M.D., Adjunct Professor of Medicine,
University of California, San Francisco, CA |
 |
The taxanes (along with new synthetic vincas, such as vinorelbine)
have proven to be among the most active agents in the treatment
of breast cancer. Most recently paclitaxel has been evaluated in
the adjuvant setting in three randomized trials. Results from Intergroup
trial 9344 have been reported several times with consistent results.
There is a statistically significant improvement in both disease-free
and overall survival that appeared by the 1st year of follow-up.
However, an unplanned subset analysis of this study demonstrated
that all or almost all of the benefit was seen among those patients
who had receptor negative tumors and did not receive tamoxifen.
The reduction in the hazard of recurrence over six years in the
ER negative group is 29% greater than that achieved with 4 cycles
of cyclophosphamide and doxorubicin (CA) alone. NSABP B-28 was of
a similar size and design as 9344. The trials differed primarily
in the mix of patients enrolled, the use of tamoxifen in a larger
proportion of the NSABP patients, and the use of a higher dose of
paclitaxel by the NSABP. Preliminar y data were presented at the
NIH consensus conference. In the population as a whole, no significant
benefits were observed in the group that received paclitaxel, but
a differential effect of paclitaxel was seen between those who had
ER positive tumors and received tamoxifen and those with ER negative
tumors, just as in trial 9344. However, in the NSABP study the 14%
reduction in hazard of recurrence for this group was not statistically
significant. The results from both trials were confounded by the
fact that those randomized to the paclitaxel arm of the study received
6 months of therapy while those given only CA had only 3 months.
A smaller randomized trial in which the duration of therapy was
constant in both arms was conducted by M.D. Anderson. This study
demonstrated a benefit from using paclitaxel, but the number of
events available from this study were too few to reliably detect
a benefit of the size seen in trial 9344. Important questions about
the use of taxanes are currently under evaluation by the cooperative
groups. These include the relative efficacy of paclitaxel and docetaxel
and the ideal dose-schedule, weekly or every 3 weeks.
| Herceptin as single agent and/or combination
with cytotoxic or hormonal agents Debu Tripathy, M.D., UCSF
Carol Franc Buck Breast Care Center, University of California,
San Francisco, CA |
 |
The neu oncogene was initially identified over 20 years ago in
rat carcinogen-induced neural tumors. The human homologue of this
gene, HER2/neu, encodes a transmembrane tyrosine kinase receptor,
which belongs to a family of receptors that are involved in numerous
functions including embryological development and cell growth. Amplification
of the HER2/neu gene and overexpression of the protein product was
found to present in 20-30 percent of primary breast tumors and accompanied
by a worse outcome, suggesting that this may represent a potential
therapeutic target. Monoclonal antibodies to HER2/neu were shown
to inhibit HER2/neu-expressing breast cancer cells and to act synergistically
with certain chemotherapies such as taxanes and platinum agents.
A humanized anti-HER2 antibody, Herceptin, has now been tested in
small and larger clinical trials and demonstrated activity as shown
below:
Table 1 - Phase II trials Herceptin for Breast cancer
|
Therapy
|
Prior Chemotherapy for Advanced Disease
|
N
|
Response Rate
|
Median Response
Duration
|
Median Time to
Disease Progression
|
| Herceptin |
Any
|
46
|
11%
|
6.6 mo
|
5.1 mo
|
| Herceptin |
1 or 2 prior regimens
|
39
|
24%
|
5.3 mo
|
Not reported
plus Cisplatin
|
| Herceptina |
1 or 2 prior regimens
|
222
|
15%
|
9.1 mo
|
3.0 mo
|
| Herceptinb |
None
|
114
|
26%
|
16.6 mo
|
3.5 mo
|
a Herceptin was given as a loading dose of 4 mg/kg followed by
2 mg/kg intravenously every week
b Patients were randomized to 4 mg/kg followed by 2 mg/kg intravenously
every week vs 8 mg/kg followed by 4 mg/kg every week
Table 2 Randomized Trial of Chemotherapy vs. Chemotherapy plus
Herceptin
|
Treatment
|
N
|
Median Time to
|
p value
Disease Progression
|
Median Survival
|
p value
|
| Chemotherapy |
234
|
4.6 mo
|
0.001
|
20.3 mo
|
0.046
|
| Chemotherapy + Herceptin |
235
|
7.4 mo
|
|
25.1 mo
|
|
| AC |
138
|
6.1 mo
|
0.001
|
21.4 mo
|
0.16
|
| AC + Herceptin |
143
|
7.8 mo
|
|
26.8 mo
|
|
| Paclitaxel |
96
|
3.0 mo
|
0.001
|
18.4 mo
|
0.17
|
| Paclitaxel+ Herceptin |
92
|
6.9 mo
|
|
22.1 mo
|
|
|
Treatment
|
N
|
Response Rate
|
p value
|
Median Response
Duration
|
p value
|
| Chemotherapy |
234
|
32%
|
0.001
|
6.1 mo
|
0.001
|
| Chemotherapy + Herceptin |
235
|
50%
|
|
9.1 mo
|
|
| AC |
138
|
42%
|
0.02
|
6.7 mo
|
0.005
|
| AC + Herceptin |
143
|
56%
|
|
9.1 mo
|
|
| Paclitaxel |
96
|
17%
|
0.001
|
4.5 mo
|
0.01
|
| Paclitaxel+ Herceptin |
92
|
41%
|
|
10.5 mo
|
|
AC= Anthracycline plus cyclophosphamide
Thus, this biologically targeted therapy is one of the few that
have been shown to improve survival in metastatic breast cancer.
However, cardiomyopathy, which is felt to be due to modulation of
HER2-mediated signaling in myocytes has been observed, especially
in combination with anthracyclines. Otherwise, therapy is generally
well tolerated. Herceptin is currently indicated in combination
with paclitaxel for first line therapy of HER2-positive metastatic
breast cancer and as a single agent for refractory disease. Clinical
trials have shown promising results with Herceptin in combinations
with other agents such as weekly paclitaxel and vinorelbine. Other
agents and hormonal combinations are also being studied.
The optimal way to determine HER2/neu tumor status and likelihood
of response to Herceptin remains somewhat controversial. Immunohistochemical
staining may be falsely negative due to tissue fixation but can
also be falsely positive. Direct analysis of gene amplification
using fluorescence in situ hybridization (FISH) may allow for a
more accurate assessment of HER2 status and for better patient selection.
Additionally, many patients with truly HER2-positive tumors do not
respond to Herceptin for reasons that are not known, and there is
active research ongoing to identify protein and genetic markers
that will better predict responsiveness to therapy.
Currently, large multi-center trials are ongoing for patient with
early stage breast cancer in both Europe and the United States.
In general, eligible patients have HER2-positive tumors (3+ by immunohistochemical
staining or positive by FISH) and positive axillary nodes. Most
trials are using anthracycline and cyclophosphamide therapy followed
by a taxane given with or without Herceptin and one trial is also
testing Herceptin with a platinum agent and a taxane. In these trials,
the benefit would need to exceed any cardiotoxicity, so very close
monitoring for these events is planned. Detailed centralized tissue
testing is also built in so that further correlations between tissue
markers and clinical benefit can be made.
References
Shih C, Padhy LC, Murray M, et al. Transforming genes of carcinomas
and neuroblastomas introduced into mouse fibroblasts. Nature 290:26l,
l98l.
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation
of relapse and survival with amplification of the HER-2/neu oncogene.
Science 235:l77, l987.
Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of
weekly intravenous recombinant humanized anti-p185HER2/neu monoclonal
antibody in patients with HER2/neu overexpressing metastatic breast
cancer. J Clin Oncol 14:737, 1996.
Cobleigh M, Vogel C, Tripathy D, et al. Multinational study of
efficacy and safety of humanized anti-HER2 monoclonal antibody
in women who have HER2-overexpressing metastatic breast cancer
that has progressed after chemotherapy for metastatic disease.
J Clin Oncol 17:2639, 1999.
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy
plus a monoclonal antibody against HER2 for metastatic breast
cancer that overexpresses HER2. N Engl J Med 344:783, 2001.
Seidman AD; Fornier MN; Esteva FJ, et al. Weekly trastuzumab
and paclitaxel therapy for metastatic breast cancer with analysis
of efficacy by HER2 immunophenotype and gene amplification. J
Clin Oncol 2001; 19:2587-95.
Burstein HJ, Kuter I, Campos SM et al. Clinical activity of trastuzumab
and vinorelbine in women with HER2-overexpressing metastatic breast
cancer. J Clin Oncol 2001; 19:2722-30.
| Microdissected breast cancer biopsied before,
during and after Hereptin and Taxol therapy Emmanuel Petricoin,
M.D., Co-Director, FDA-NCI Clinical Proteomics Program, Bethesda,
MD |
 |
While the underlying cause of human cancer lies mainly within multiple
genetic mutations, at a functional level cancer is a proteomic disease.
Derangements in signal pathway activation, the cellular “circuitry”,
ultimately underpin aberrant cellular growth and metastasis. However,
the exact identification of the rewiring of the protein network
has never been elucidated in actual human cancer cells in tissue,
with most information being gleaned in cell culture and animal model
studies. Treatment with new classes of molecular targeted therapeutics
such as IRESSA(r), GLEEVEC(r) or HERCEPTIN(r), requires the presence
of over-expressed receptors or activated signaling molecules for
therapeutic success. Ultimately, realization for a future in which
true patient-tailored therapy is realized will rely on new proteomic
approaches to discover the states of signal pathway activation within
the patients tumor, and the effect of therapy not just on the proposed
target but also on the pathway in which the target resides. We therefore
employed Laser Capture Microdissection obtain an in vivo pattern
of pathway activation within cancer cases that revealed patient-specific
activation patterns regardless of over-expression and activation
of c-ErbB2. Our analysis in primary tissue highlights the complexity
of cellular signaling that occurs within human cancer cells and
underscores the importance of phosphoprotein screening in clinical
diagnostics. Finally, we extend our analysis to multiplexed signal
pathway profiling in human biopsy material obtained from patients
before, during and after HERCEPTIN(r) treatment and show the Akt
pro-survival pathway is repressed in the treated samples.
| Capecitabine: lessons learned and future
directions Kathy D. Miller, M.D., Associate Professor of Medicine,
Indiana University, Indianapolis, IN |
 |
Capecitabine is an oral fluoropyrimidine prodrug activated by a
three-step metabolic process to 5-fluorouracil. As malignant tissue
typical has higher thymidine phosphorylase activity, the end result
is higher concentrations of 5-fluorouracil in tumor relative to
surrounding normal tissue. In a multicenter phase II trial, objective
responses were seen in 20% of patients who had had prior anthracycline-
and taxane-based therapy leading to FDA approval. Side effects are
similar to infusional 5-FU with diarrhea and hand-foot syndrome
being the most frequent; myelosuppression and alopecia are uncommon.
Combination with several existing agents is being explored. Recent
exploration of alternate doses and schedules have limited toxicity
and increased the use of this important agent. Two adjuvant trials
including capecitabine are planned.
| Taxotere + Xeloda in Metastatic Breast Cancer
(MBC) Stephen E. Jones, M.D., Baylor-Sammons Cancer Center,
Dallas, Texas, and US Oncology Research, Houston, TX |
 |
Background and rationale
There are many treatments for MBC including a variety of chemotherapeutic
agents, most often employed as single agents. These include the
taxanes, capecitabine (Xeloda), Navelbine and others. The goals
of treatment are palliation and if possible prolongation of life.
Two of the commonly used drugs are Taxotere and Xeloda, the latter
of which is a prodrug, converted to 5-FU in tumor tissue by the
enzyme thymidine phosphorylase (TP). TP is upregulated by the taxanes
so there is a strong indication to evaluate the 2 agents together
in MBC.
In the largest phase II study of Xeloda in 163 patients with MBC,
the objective response (ORR) rate was 20% and 43% had stable disease.(1)
With respect to Taxotere in MBC, response rates as high as 60% have
been reported. In a randomized trial against doxorubicin Taxotere,
produced an ORR of 48% compared to 33% for doxorubicin (p=0.008)
as well as longer overall survival (OS).(2) Study Plan: The above
rationale led to a randomized phase III trial of Taxotere (100 mgs/m2
IV q 3 weeks) versus Taxotere (75 mgs/m2 IV q 3 weeks) plus oral
Xeloda (1250 mgs/m2 BID days 1-14) every 3 weeks in patients with
MBC.
The results are summarized below: (3)
| |
|
TX (255)
|
T (256)
|
|
|
|
| ORR (%) |
42
|
|
30
|
|
p=0.006
|
|
| Stable >6 mos. (%) |
|
41
|
|
29
|
|
p=0.004
|
| TTP (median, mos) |
6.1
|
|
4.2
|
|
p=0.001
|
|
| OS |
|
13.7
|
|
11.1
|
|
p=0.01
|
Toxicity in this study will be described. The results of this study
led to FDA approval in 2001 of this XT regimen for patients with
MBC.
References
- Blum et al JCO 17:485, 1999.
- Chan et al JCO 17:2341, 1999.
- O’Shaughnessy et al, SABCS 2000 and JCO (in press, 2002).
| New Drug Developments Daniel Hayes, M.D.,
Associate Professor of Medicine, Georgetown University Medical
Center, Washington, DC |
 |
Over the last decade, several new drugs have been approved for
treatment of breast cancer. These fall into four categories (bisphosphonates:
pamidrondate; chemotherapy: capecitabine, epirubicin, paclitaxel,
docetaxel; hormone therapy: anastrazole, exemestane, goserelin,
letrozole, tamoxifen (new indications: DCIS, prevention), toremifene;
and anti-HER2 therapy (trastuzumab). Several other new drugs have
activity against breast cancer and have been commonly incorporated
into treatment in the metastatic setting (liposomal doxorubicin,
gemcitabine, vinorelbine).
Several new approaches have been and are being studied now. These
include, broadly, EGFR family tyrosine kinase inhibitors (iressa,
CI1033, others), anti-angiogenic agents (anti-VEGF, VEGF-r tyrosine
kinase inhibitor, TNP470, MMPIs, others), anti-proliferative agents
(CCI 779, others), and immunotherapeutic approaches. Another broad
class of agents includes the anti-sense oligonucleotidcs (ASO).
ASO against PKC and against bcl-2 are both in clinical trials. Each
of these is in phase I or II trials now. Of these, anti-VEGF appears
to be the most promising in terms of currently available data and
the status of its development.
A major issue is phase II trial design. Obviously, major responses
do not require new designs, but many of these agents may be more
“cytostatic” and may not induce responses. Therefore,
classic Phase II trial designs will result in false positive results.
Newer Phase II trial designs use either “failure to progress”
and/or surrogate biomarkers as endpoints to indicated some element
of activity.
Back | Top of Page


|
|



