
 |
||||||||||||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 2-3
![]() DR LOVE: What do we know about the benefits of hormonal therapy for
patients with a high Oncotype DX recurrence score?
DR LOVE: What do we know about the benefits of hormonal therapy for
patients with a high Oncotype DX recurrence score?
![]() DR GEYER: We haven’t published the manuscript yet, but it appears that these
patients don’t receive much benefit from tamoxifen, even though they are in the
subset categorized as hormone receptor-positive. We observed a clear benefit
from tamoxifen for the patients in the low- and intermediate-risk categories.
DR GEYER: We haven’t published the manuscript yet, but it appears that these
patients don’t receive much benefit from tamoxifen, even though they are in the
subset categorized as hormone receptor-positive. We observed a clear benefit
from tamoxifen for the patients in the low- and intermediate-risk categories.
I don’t believe people should take that to mean that hormone therapy confers no benefit for patients characterized as being at high risk by Oncotype DX.
It does mean that those women who don’t want chemotherapy and hope to receive benefit from hormonal therapy may not be receiving as much benefit, on average, as the broader population of patients with hormone receptor-positive disease.
![]() DR LOVE: Can you discuss the MammaPrint assay?
DR LOVE: Can you discuss the MammaPrint assay?
![]() DR GEYER: This assay requires frozen specimens, so to use it, you have to
alter your practice. It was developed to dichotomize patients into low-risk or
high-risk categories, the idea being that patients at low risk would not need
any therapy.
DR GEYER: This assay requires frozen specimens, so to use it, you have to
alter your practice. It was developed to dichotomize patients into low-risk or
high-risk categories, the idea being that patients at low risk would not need
any therapy.
The studies were conducted with mixed populations of patients with ER-positive and ER-negative disease. The low-risk group has a rate of recurrence of approximately 10 percent, so you would certainly still administer hormonal therapy to some patients.
I don’t see what MammaPrint has to offer that Oncotype DX doesn’t already provide. The Oncotype DX assay can be performed on paraffin-embedded tissue, so you don’t have to alter your practice patterns.
If you’re going to use MammaPrint, you have to collect tissue from every patient, whether or not you know her nodal status, hormone receptor status and HER2 status. At least with Oncotype DX you can wait to determine the HER2 status because with HER2 amplification, you don’t need the assay.
Tracks 4-5, 8
![]() DR LOVE: The patient you are presenting in the American Society of
Breast Surgeons meeting was perimenopausal. How are you approaching
the choice of hormone therapy with postmenopausal patients?
DR LOVE: The patient you are presenting in the American Society of
Breast Surgeons meeting was perimenopausal. How are you approaching
the choice of hormone therapy with postmenopausal patients?
![]() DR GEYER: Usually, for a woman who is clearly postmenopausal, who does
not have significant, chronic musculoskeletal problems and who has reasonably
well-preserved bone density, I recommend an aromatase inhibitor.
DR GEYER: Usually, for a woman who is clearly postmenopausal, who does
not have significant, chronic musculoskeletal problems and who has reasonably
well-preserved bone density, I recommend an aromatase inhibitor.
I do try to help women understand that the differences between the aromatase inhibitors and tamoxifen are small — the absolute further incremental benefit is small — so what’s important is that we find a hormone therapy that the patient can tolerate for five years.
All things being equal, the aromatase inhibitors do appear to be more effective, particularly when the disease is more aggressive. The more you’re worried about the patient, the more you want to treat with an aromatase inhibitor.
![]() DR LOVE: It’s interesting that you raise the issue of trying to find a therapy
the patient can receive for five years because I’ve seen a significant change in
how people view the long-term history of breast cancer. I notice more sensitivity
to what’s happening not only later in the first five years but also in years
five to 10, 10 to 15 and beyond.
DR LOVE: It’s interesting that you raise the issue of trying to find a therapy
the patient can receive for five years because I’ve seen a significant change in
how people view the long-term history of breast cancer. I notice more sensitivity
to what’s happening not only later in the first five years but also in years
five to 10, 10 to 15 and beyond.
![]() DR GEYER: Without question, the MA17 data have shown us that long-term
hormonal therapy does help control disease (Goss 2005a, 2005b). The trial
ended early because the effects were observed quickly and the magnitude of
the effects was greater than anticipated.
DR GEYER: Without question, the MA17 data have shown us that long-term
hormonal therapy does help control disease (Goss 2005a, 2005b). The trial
ended early because the effects were observed quickly and the magnitude of
the effects was greater than anticipated.
Another interesting observation was that patients who were on the placebo arm crossed over to letrozole and then experienced treatment benefit. Those were amazing data.
Even with the several-year break from receiving hormonal therapy, the reinstitution of therapy drove the rates of recurrence down. So the MA17 data gave oncologists a stronger sense that hormone-dependent breast cancer is the chronic disease we’ve talked about.
![]() DR LOVE: How does that translate to your own clinical practice in terms of
starting an aromatase inhibitor for a patient who’s been off tamoxifen for a few
years?
DR LOVE: How does that translate to your own clinical practice in terms of
starting an aromatase inhibitor for a patient who’s been off tamoxifen for a few
years?
![]() DR GEYER: Certainly, a patient who had positive nodes and was tolerating
medication well but then was stopped, in a sense arbitrarily, because the available
data suggested she should stop is somebody with whom you may want to
revisit and share the data.
DR GEYER: Certainly, a patient who had positive nodes and was tolerating
medication well but then was stopped, in a sense arbitrarily, because the available
data suggested she should stop is somebody with whom you may want to
revisit and share the data.
![]() DR LOVE: That approach is exactly what I hear a lot of support for because
we don’t know the answer. Can you make the argument that if a surgeon is
routinely following up on a patient who had an ER-positive tumor and did
not receive an aromatase inhibitor, a red flag should appear on the chart?
DR LOVE: That approach is exactly what I hear a lot of support for because
we don’t know the answer. Can you make the argument that if a surgeon is
routinely following up on a patient who had an ER-positive tumor and did
not receive an aromatase inhibitor, a red flag should appear on the chart?
![]() DR GEYER: It’s a reasonable question for a surgeon who continues to follow
patients for years. Frequently, patients stop seeing their medical oncologist
when they finish therapy, and they may not have this discussion. This is an
instance in which it’s useful for breast surgeons to be aware of the data and
consider the issue of further therapy with their patients.
DR GEYER: It’s a reasonable question for a surgeon who continues to follow
patients for years. Frequently, patients stop seeing their medical oncologist
when they finish therapy, and they may not have this discussion. This is an
instance in which it’s useful for breast surgeons to be aware of the data and
consider the issue of further therapy with their patients.
Track 13
![]() DR LOVE: Could you summarize the data with adjuvant trastuzumab for
patients with HER2-positive tumors?
DR LOVE: Could you summarize the data with adjuvant trastuzumab for
patients with HER2-positive tumors?
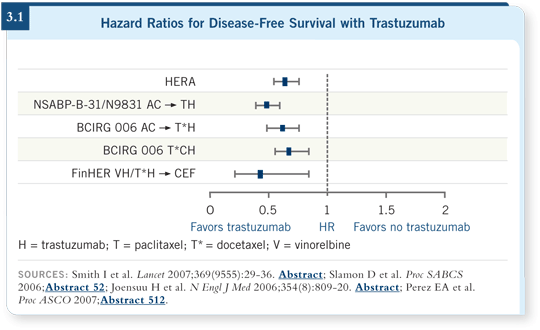
![]() DR GEYER: Four large adjuvant studies were initiated to determine whether
adding trastuzumab to chemotherapy could improve the outcome for women
with HER2-positive breast cancer ( Joensuu 2006; Perez 2007; Piccart-Gebhart 2005; Romond 2005; Slamon 2005, 2006; Smith 2007; [3.1]).
DR GEYER: Four large adjuvant studies were initiated to determine whether
adding trastuzumab to chemotherapy could improve the outcome for women
with HER2-positive breast cancer ( Joensuu 2006; Perez 2007; Piccart-Gebhart 2005; Romond 2005; Slamon 2005, 2006; Smith 2007; [3.1]).
Across all trials, when trastuzumab was administered with chemotherapy, a substantial — 40 to 50 percent — reduction in risk of recurrence occurred quickly. All these trials reported early because the effects were greater than anticipated. So a striking consistency of benefit is evident when you add trastuzumab, making the nuances of chemotherapy appear less important.
| Table of Contents | Top of Page |
Editor:
Neil Love, MD
Interviews
Melvin J Silverstein, MD
- Select publications
David M Hyams, MD
- Select publications
Charles E Geyer Jr, MD
- Select publications
Hope S Rugo, MD
- Select publications
Highlights of a CME Symposium Held in Conjunction
with The American Society of Breast Surgeons Eighth
Annual Meeting
- Select publications
A CME Audio Series and Activity