
 |
||||||||||||||

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-3

![]() DR LOVE: How did you approach the discussion of further endocrine
therapy with this patient?
DR LOVE: How did you approach the discussion of further endocrine
therapy with this patient?
![]() DR RUGO: At that point, she was six and a half years from her diagnosis of
node-positive disease. Since she started therapy, the data have changed. We
now understand more about the natural history of hormone receptor-positive
breast cancer.
DR RUGO: At that point, she was six and a half years from her diagnosis of
node-positive disease. Since she started therapy, the data have changed. We
now understand more about the natural history of hormone receptor-positive
breast cancer.
We understand that if approximately 50 percent of recurrences occur after five years, then a substantial residual risk remains. The residual risk for those patients will not be decreased much by the one or two years that she was off therapy. It’s clear that hormonal therapy might provide additional benefit.
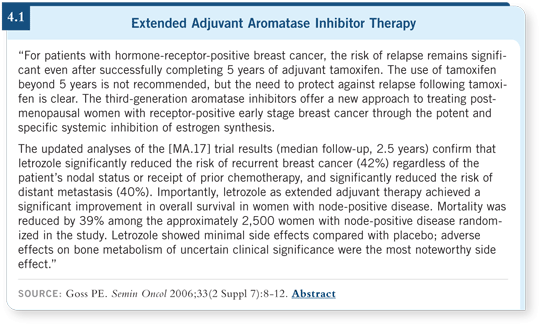
Data from MA17 demonstrated that women who allocated themselves to letrozole after unblinding did well compared to women who did not (Robert 2006; [4.1]). This suggests that if you start hormone therapy — even eight years after diagnosis — you may affect patients. For some patients who didn’t receive tamoxifen early on, you can still start an aromatase inhibitor at a later time if they have higher-risk disease.
![]() DR LOVE: Was she surprised when you broached the issue of hormone
therapy, considering she had been doing so well for the last seven years?
DR LOVE: Was she surprised when you broached the issue of hormone
therapy, considering she had been doing so well for the last seven years?
![]() DR RUGO: Yes. Women want to believe that five years is a magic time period.
They don’t want to hear that 50 percent of their recurrence risk — and, in
truth, more than 50 percent of their risk of dying — lies ahead of them.
DR RUGO: Yes. Women want to believe that five years is a magic time period.
They don’t want to hear that 50 percent of their recurrence risk — and, in
truth, more than 50 percent of their risk of dying — lies ahead of them.
I have a patient who had a 1.9-cm, intermediate-grade, node-negative tumor. She received CMF, went into menopause in her mid-thirties and then received five years of tamoxifen. When she reached her five-year point, I said, “You are in menopause and could take an aromatase inhibitor.” She said, “I had a Stage I tumor, so I don’t believe that’s worthwhile.” Three years later, she had metastatic disease to her bone.
Without concrete data, which we’ll never have, I tailor the duration of the aromatase inhibitor therapy to the extended risk. I say, “Okay, you had node-negative disease, but an aromatase inhibitor adds to tamoxifen in every single trial that’s available. Why don’t we use two or three years of an aromatase inhibitor?”
If a patient has higher-risk disease, it brings up the next point: What do you do with a patient at particularly high risk who’s been on five years of an aromatase inhibitor from diagnosis? Do you continue or stop?
In some ways, it’s like stopping trastuzumab at one year for a patient who has inflammatory breast cancer and positive margins. At five years, I often continue the aromatase inhibitor, although it is impossible to know the optimal duration.
Track 12
![]() DR LOVE: Can you discuss the issue of HER2 testing?
DR LOVE: Can you discuss the issue of HER2 testing?
![]() DR RUGO: It’s important to have adequate tumor tissue to make these
assessments. I have had patients come in with inadequate tissue sampling
— primarily women who underwent a fine-needle aspiration for diagnosis —
who then received neoadjuvant therapy and underwent surgery, and nobody
rechecked any of the tumor markers.
DR RUGO: It’s important to have adequate tumor tissue to make these
assessments. I have had patients come in with inadequate tissue sampling
— primarily women who underwent a fine-needle aspiration for diagnosis —
who then received neoadjuvant therapy and underwent surgery, and nobody
rechecked any of the tumor markers.
For HER2, if an immunohistochemistry (IHC) test is performed at a low-volume institution, you always want to repeat the IHC or obtain a fluorescence in situ hybridization test to pin down whether or not the patient has HER2-positive disease. It’s critical to have that information as soon as possible in order to help with treatment decisions.
You don’t want to be treating a woman who doesn’t have HER2-positive disease with trastuzumab. However, you wouldn’t want to miss administering trastuzumab to a patient who has HER2-positive disease.
| Table of Contents | Top of Page |
Editor:
Neil Love, MD
Interviews
Melvin J Silverstein, MD
- Select publications
David M Hyams, MD
- Select publications
Charles E Geyer Jr, MD
- Select publications
Hope S Rugo, MD
- Select publications
Highlights of a CME Symposium Held in Conjunction
with The American Society of Breast Surgeons Eighth
Annual Meeting
- Select publications
A CME Audio Series and Activity