|

| Tracks 1-19 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Background for the combined
NSABP/NCCTG analysis |
| Track 3 |
Incorporation of the adjuvant
trastuzumab trial results into
clinical practice |
| Track 4 |
Clinical use of adjuvant trastuzumab
in elderly patients |
| Track 5 |
Concurrent versus sequential
adjuvant trastuzumab and
chemotherapy |
| Track 6 |
Delayed adjuvant trastuzumab |
| Track 7 |
Impact of adjuvant trastuzumab
on distant disease-free survival |
| Track 8 |
Impact of the risk of relapse on
management of patients with
HER2-positive, node-negative
disease |
| Track 9 |
Future adjuvant trials combining
bevacizumab with trastuzumab
in patients with HER2-positive
disease |
|
| Track 10 |
Clinical use of adjuvant docetaxel/
carboplatin/trastuzumab |
| Track 11 |
Adjuvant trastuzumab for patients
with reduced ejection fraction
following AC |
| Track 12 |
NSABP neoadjuvant trastuzumab
trial |
| Track 13 |
Use of adjuvant trastuzumab prior
to the release of the trial results |
| Track 14 |
Combining trastuzumab with
other biologic agents |
| Track 15 |
Clinical trial concept of trastuzumab
plus bevacizumab |
| Track 16 |
Disease-free survival as the
primary endpoint for adjuvant
trials |
| Track 17 |
Cardiac safety analysis in the
adjuvant trastuzumab trials |
| Track 18 |
Clinical trials mechanisms |
| Track 19 |
Perspectives on future directions
in clinical research |
|
|
Select Excerpts from the Interview
 Track 2 Track 2
 DR LOVE: Can you discuss the rationale for the combined analysis of the
adjuvant trastuzumab trials? DR LOVE: Can you discuss the rationale for the combined analysis of the
adjuvant trastuzumab trials? |
 DR WOLMARK: Originally, the NSABP-B-31 trial was going to be an indication
trial. The NCCTG-N9831 trial was scheduled to begin later as it really
didn’t make sense to conduct the two trials concurrently in a population of
patients with node-positive disease who account for only one quarter or perhaps even less of the patient spectrum. As it turned out, by the time all of the regulatory
prerequisites were met, the two trials started almost concomitantly. DR WOLMARK: Originally, the NSABP-B-31 trial was going to be an indication
trial. The NCCTG-N9831 trial was scheduled to begin later as it really
didn’t make sense to conduct the two trials concurrently in a population of
patients with node-positive disease who account for only one quarter or perhaps even less of the patient spectrum. As it turned out, by the time all of the regulatory
prerequisites were met, the two trials started almost concomitantly.
So there were only a few months between the time the NSABP-B-31 trial
started and the time N9831 started. No one had envisioned that would happen;
it certainly wasn’t planned. The rate of accrual for a quarter of the population
with node-positive disease distributed between not only two competing studies
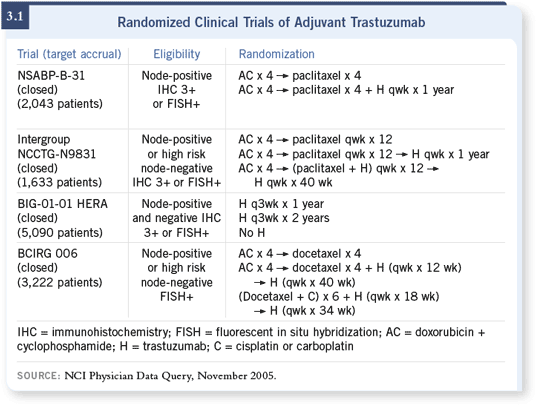
but also a third, if you include the BCIRG 006 study (3.1), was less than ideal.
It became apparent that there was a need to really maintain focus on the goal
of these trials, namely, to determine the efficacy of trastuzumab in women
with breast cancer. If we were able to answer the question sooner, that was the
right thing to do.
The combined analysis did not occur because someone decided, “We’re
going to have a look at the data.” This was a very demanding and rigorous
process for which a combined analysis plan was submitted to both the Cancer
Therapy Evaluation Program and the FDA, as it required a number of changes,
including our request to change the endpoint for analysis from overall survival
to disease-free survival.
Once the combined analysis was approved, things moved very rapidly. The
joint analysis plan was approved in January 2005, and the Data Monitoring
Committee met during the third week of April. The first interim analysis
indicated the number of requisite events had been surpassed. The requirement
was for 355 events, and we actually had 395. The prerequisite for disclosure of
the data was a p-value of 10-3, and we had a p-value of 10-13.
 DR LOVE: Do you have a sense of how much earlier we received the results
because of this combined analysis? DR LOVE: Do you have a sense of how much earlier we received the results
because of this combined analysis?
 DR WOLMARK: We saved a considerable amount of time — probably two
years. The NSABP study alone also crossed the boundaries with the proviso
that we use disease-free survival as an endpoint, which was not the primary
endpoint of the single trial analysis. DR WOLMARK: We saved a considerable amount of time — probably two
years. The NSABP study alone also crossed the boundaries with the proviso
that we use disease-free survival as an endpoint, which was not the primary
endpoint of the single trial analysis.
 DR LOVE: Just to clarify, the NSABP-B-31 trial contained two arms, and the
NCCTG-N9831 trial consisted of three arms. The two common arms were
used in the combined analysis, correct? DR LOVE: Just to clarify, the NSABP-B-31 trial contained two arms, and the
NCCTG-N9831 trial consisted of three arms. The two common arms were
used in the combined analysis, correct?
 DR WOLMARK: Precisely. The arms were so similar that not to combine them,
I believe, would have been a disservice to women with breast cancer. DR WOLMARK: Precisely. The arms were so similar that not to combine them,
I believe, would have been a disservice to women with breast cancer.
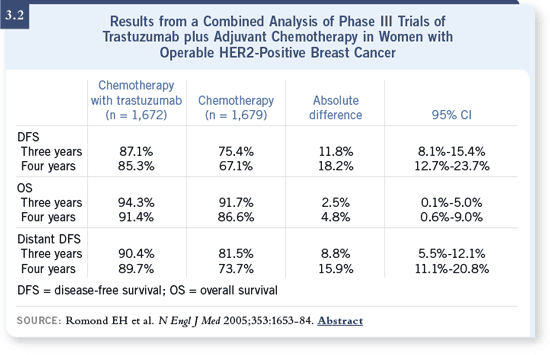
 DR LOVE: When people first heard this analysis was going to be conducted,
there were a lot of questions about whether or not this type of evaluation was
appropriate. However, after the results revealed such large differences, the
analysis was no longer questioned (3.2). What is your interpretation of what
occurred? DR LOVE: When people first heard this analysis was going to be conducted,
there were a lot of questions about whether or not this type of evaluation was
appropriate. However, after the results revealed such large differences, the
analysis was no longer questioned (3.2). What is your interpretation of what
occurred?
 DR WOLMARK: The methodology used for the combined analysis was
absolutely solid. It was the right thing to do because, in essence, the trials were
the same trial with some minor variations in the common arms. If the result
had been considerably less impressive or only marginal, I still believe the data
would have been a ref lection of what was actually happening. DR WOLMARK: The methodology used for the combined analysis was
absolutely solid. It was the right thing to do because, in essence, the trials were
the same trial with some minor variations in the common arms. If the result
had been considerably less impressive or only marginal, I still believe the data
would have been a ref lection of what was actually happening.
 Track 5 Track 5
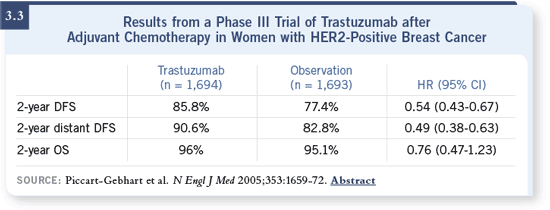
 DR LOVE: There is some confusion amongst community-based oncologists
regarding the issue of concurrent versus sequential trastuzumab/chemotherapy because the HERA study data demonstrate positive results
in patients who received trastuzumab after chemotherapy (Piccart-
Gebhart 2005; [3.3]). Essentially, there was no benefit in the sequential
treatment arm of the NCCTG-N9831 trial. How do you interpret
those findings? DR LOVE: There is some confusion amongst community-based oncologists
regarding the issue of concurrent versus sequential trastuzumab/chemotherapy because the HERA study data demonstrate positive results
in patients who received trastuzumab after chemotherapy (Piccart-
Gebhart 2005; [3.3]). Essentially, there was no benefit in the sequential
treatment arm of the NCCTG-N9831 trial. How do you interpret
those findings? |
 DR WOLMARK: The only test of concomitant versus sequential therapy was in
the NCCTG-N9831 trial. When you look at the curves in the comparisons
of both treatment arms to the control, I do not believe that one can remain
neutral. The concomitant arm had a hazard rate that fell in line with what
we’re seeing in the other trials, including BCIRG 006 and the combined
analysis. However, this is not true of the comparison between the control and
sequential arm of N9831, which was associated with a hazard rate of 0.87
(p = 0.29). DR WOLMARK: The only test of concomitant versus sequential therapy was in
the NCCTG-N9831 trial. When you look at the curves in the comparisons
of both treatment arms to the control, I do not believe that one can remain
neutral. The concomitant arm had a hazard rate that fell in line with what
we’re seeing in the other trials, including BCIRG 006 and the combined
analysis. However, this is not true of the comparison between the control and
sequential arm of N9831, which was associated with a hazard rate of 0.87
(p = 0.29).
The comparison of concomitant treatment with trastuzumab versus sequential
treatment with trastuzumab was associated with a hazard rate of 0.64, which
was significant (p = 0.01; [Perez 2005a]). It’s not inappropriate for a medical
oncologist to look at those data and say they are more impressed with data
from the concomitant use of trastuzumab.
Are the results from NCCTG-N9831 inconsistent with the HERA data? Not
necessarily. If you look at the hazard in the taxane-treated population in the
HERA trial, the least impressive hazard rate occurred in those patients. So the
question is whether or not this occurred because they received more cycles of
chemotherapy, which delayed the administration of trastuzumab.
The data are not necessarily inconsistent with one another. I think these trials
all show there is clearly a benefit of trastuzumab when given concomitantly
with chemotherapy, and there may be a benefit sequentially.
 Track 6 Track 6
 DR LOVE: What are your thoughts on the issue of delayed trastuzumab in
patients who have received chemotherapy in the recent past — six months
to two years ago. We’ve already been sensitized to this issue through our
experience with the aromatase inhibitors and the time course of disease
recurrence. Do you believe it makes sense to look at the risk of recurrence
in relation to the continuum of disease course and assume that risk might
be decreased if you administer trastuzumab, even if it is delayed? DR LOVE: What are your thoughts on the issue of delayed trastuzumab in
patients who have received chemotherapy in the recent past — six months
to two years ago. We’ve already been sensitized to this issue through our
experience with the aromatase inhibitors and the time course of disease
recurrence. Do you believe it makes sense to look at the risk of recurrence
in relation to the continuum of disease course and assume that risk might
be decreased if you administer trastuzumab, even if it is delayed? |
 DR WOLMARK: I think a conditional probability might be a good thing to
utilize as a guide to determine whether trastuzumab should or should not be
used in a delayed fashion. Would you administer trastuzumab to a patient with
five positive nodes, who has completed chemotherapy a year ago knowing or,
more specifically, not knowing what the effect is going to be at that point?
The majority of clinicians would make that decision on an individual basis,
and I would also. DR WOLMARK: I think a conditional probability might be a good thing to
utilize as a guide to determine whether trastuzumab should or should not be
used in a delayed fashion. Would you administer trastuzumab to a patient with
five positive nodes, who has completed chemotherapy a year ago knowing or,
more specifically, not knowing what the effect is going to be at that point?
The majority of clinicians would make that decision on an individual basis,
and I would also.
 Track 7 Track 7
 DR LOVE: There has been a lot of attention on the distant disease-free
survival curve, which was dramatically better with trastuzumab. What are
your thoughts about these data? DR LOVE: There has been a lot of attention on the distant disease-free
survival curve, which was dramatically better with trastuzumab. What are
your thoughts about these data? |
 DR WOLMARK: When you see a curve of distant disease-free survival where
the investigational arm — namely, in those patients who have received trastuzumab
— is flat, it’s dramatic, particularly so early on in follow-up (3.4). Does
this mean that tumor cells have been eradicated? That’s the great hope. DR WOLMARK: When you see a curve of distant disease-free survival where
the investigational arm — namely, in those patients who have received trastuzumab
— is flat, it’s dramatic, particularly so early on in follow-up (3.4). Does
this mean that tumor cells have been eradicated? That’s the great hope.
For distant disease-free survival, we are seeing a difference of 90 percent
versus 74 percent at four years in the combined analysis — an absolute difference
of 16 percent. These results are very impressive. Have we crossed the
threshold for opportunities to treat the disease? I think we have.
 Track 10 Track 10
 DR LOVE: The initial data from BCIRG 006 have just been released.
Do you think that a future data set from this study might show equivalent
efficacy of TCH and AC DR LOVE: The initial data from BCIRG 006 have just been released.
Do you think that a future data set from this study might show equivalent
efficacy of TCH and AC TH? TH? |
 DR WOLMARK: That is the great hope, but I don’t believe it is likely. We
would have loved to have TCH show the same hazard rate as AC DR WOLMARK: That is the great hope, but I don’t believe it is likely. We
would have loved to have TCH show the same hazard rate as AC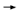 TH
— that would have made everything much more simple. You would have a
noncardiotoxic regimen that shows efficacy in the same range as an anthracycline-
containing regimen, so that would have rapidly become the preferred
regimen. TH
— that would have made everything much more simple. You would have a
noncardiotoxic regimen that shows efficacy in the same range as an anthracycline-
containing regimen, so that would have rapidly become the preferred
regimen.
According to the press release (BCIRG 2005), the relative reduction in risk of
relapse for AC TH was 51 percent and was 39 percent for TCH, so we can’t
write eulogies for the AC TH was 51 percent and was 39 percent for TCH, so we can’t
write eulogies for the AC TH regimen, and we ought not. On the other
hand, one is certainly not eliminating TCH as a template to which bevacizumab
may be added. It is not unreasonable to consider that as a possibility. TH regimen, and we ought not. On the other
hand, one is certainly not eliminating TCH as a template to which bevacizumab
may be added. It is not unreasonable to consider that as a possibility.
 DR LOVE: It’s going to be interesting to see how oncologists and clinical
investigators apply these findings to the clinical setting. Do you think it would
be reasonable to consider using nonprotocol TCH based on the current data,
particularly in the patient at lower risk or the older patient? The regimen
clearly has efficacy. DR LOVE: It’s going to be interesting to see how oncologists and clinical
investigators apply these findings to the clinical setting. Do you think it would
be reasonable to consider using nonprotocol TCH based on the current data,
particularly in the patient at lower risk or the older patient? The regimen
clearly has efficacy.
 DR WOLMARK: Medical oncologists are going to use the TCH regimen in
a reasonable and logical way, as they should. The data that were disclosed
relative to hazard rates from BCIRG 006 indicate that TCH is an effective
regimen. DR WOLMARK: Medical oncologists are going to use the TCH regimen in
a reasonable and logical way, as they should. The data that were disclosed
relative to hazard rates from BCIRG 006 indicate that TCH is an effective
regimen.
We would have liked for the hazard rate to have been the same as AC TH, but it wasn’t. We have seen a benefit when trastuzumab is added to the
regimen, so if you have a patient who has cardiac compromise and you don’t
wish to use doxorubicin, I certainly think that TCH is a reasonable alternative. TH, but it wasn’t. We have seen a benefit when trastuzumab is added to the
regimen, so if you have a patient who has cardiac compromise and you don’t
wish to use doxorubicin, I certainly think that TCH is a reasonable alternative.
 Track 11 Track 11
 DR LOVE: Can you comment on the issue of monitoring cardiac toxicity?
A common question is how to treat the patient who has a drop in ejection
fraction after receiving AC. Can you discuss what was observed in the
NSABP trial and how that translates into clinical practice? DR LOVE: Can you comment on the issue of monitoring cardiac toxicity?
A common question is how to treat the patient who has a drop in ejection
fraction after receiving AC. Can you discuss what was observed in the
NSABP trial and how that translates into clinical practice? |
 DR WOLMARK: With AC alone, we saw a significant proportion of patients
with decreases in ejection fraction. For those patients, I think medical oncologists
will be making their decisions, which are going to be mainly driven by
risk of recurrence. The higher the risk, the greater the likelihood that the
patient is going to be treated. People are going to be innovative in the way
they interpret ejection fraction or the algorithm they use. DR WOLMARK: With AC alone, we saw a significant proportion of patients
with decreases in ejection fraction. For those patients, I think medical oncologists
will be making their decisions, which are going to be mainly driven by
risk of recurrence. The higher the risk, the greater the likelihood that the
patient is going to be treated. People are going to be innovative in the way
they interpret ejection fraction or the algorithm they use.
 Track 12 Track 12
 DR LOVE: Can you talk about the NSABP-B-41 neoadjuvant trial, which
is being designed for patients with HER2-positive tumors? DR LOVE: Can you talk about the NSABP-B-41 neoadjuvant trial, which
is being designed for patients with HER2-positive tumors? |
 DR WOLMARK: We were all certainly focused on Aman Buzdar’s neoadjuvant
study, which was a small trial indicating that paclitaxel followed by FEC with
concomitant trastuzumab was associated with remarkable pCR rates (Buzdar
2005). This study caught our attention, so we would like to test that regimen
in a larger clinical trial. That is what NSABP-B-41 is going to test: the Buzdar
regimen compared to a more traditional sequential regimen of FEC followed
by a taxane with trastuzumab. DR WOLMARK: We were all certainly focused on Aman Buzdar’s neoadjuvant
study, which was a small trial indicating that paclitaxel followed by FEC with
concomitant trastuzumab was associated with remarkable pCR rates (Buzdar
2005). This study caught our attention, so we would like to test that regimen
in a larger clinical trial. That is what NSABP-B-41 is going to test: the Buzdar
regimen compared to a more traditional sequential regimen of FEC followed
by a taxane with trastuzumab.
 DR LOVE: What is the endpoint of the study? DR LOVE: What is the endpoint of the study?
 DR WOLMARK: The primary endpoint is pathologic complete response (pCR).
We want to see if the pCR rate from the Buzdar study can be duplicated.
Cardiac safety, of course, is another endpoint. DR WOLMARK: The primary endpoint is pathologic complete response (pCR).
We want to see if the pCR rate from the Buzdar study can be duplicated.
Cardiac safety, of course, is another endpoint.
 DR LOVE: What is the postsurgical therapy going to be in the B-41 study? DR LOVE: What is the postsurgical therapy going to be in the B-41 study?
 DR WOLMARK: Patients are going to receive trastuzumab for one year. Duplicating
the Buzdar results will be a major step. Then — assuming that we do
see that level of pCR — we will analyze the components of the regimen that
led to the response. The Buzdar regimen was novel in that trastuzumab was
administered concomitantly with both the taxane and the FEC. DR WOLMARK: Patients are going to receive trastuzumab for one year. Duplicating
the Buzdar results will be a major step. Then — assuming that we do
see that level of pCR — we will analyze the components of the regimen that
led to the response. The Buzdar regimen was novel in that trastuzumab was
administered concomitantly with both the taxane and the FEC.
 DR LOVE: Can you discuss the NSABP-B-40 neoadjuvant study of three
different arms of preoperative neoadjuvant chemotherapy? DR LOVE: Can you discuss the NSABP-B-40 neoadjuvant study of three
different arms of preoperative neoadjuvant chemotherapy?
 DR WOLMARK: The novelty of B-40 is that we’re using pCR as an endpoint
with an emphasis on developing a molecular taxonomy to determine whether
or not we can characterize patients who obtain a pCR as a surrogate marker to
measure outcome. There is a definite interest in tissue collection in the B-41
study. DR WOLMARK: The novelty of B-40 is that we’re using pCR as an endpoint
with an emphasis on developing a molecular taxonomy to determine whether
or not we can characterize patients who obtain a pCR as a surrogate marker to
measure outcome. There is a definite interest in tissue collection in the B-41
study.
Disease-free survival and overall survival are not endpoints for the NSABPB-
40 protocol. We view it as a new mechanism to test promising agents in
the neoadjuvant setting, and we think it is an appropriate direction to pursue,
particularly with the number of agents that are available and the limited
resources, both from a support standpoint and a population standpoint.
Select publications
|

