Section 6
ATAC Trial: Arimidex vs Tamoxifen vs
Combination
UPDATE
ON ACCRUAL
I chair
the steering committee and have seen this trial through from
back-of-an-envelope discussions in a pub four years ago to
completing accrual of 9,300 patients in record time. It’s
an amazing international collaboration — particularly
an Anglo-American collaboration. The interim analysis from
the Data Monitoring Committee at the end of last year advised
us that there was no excess of predetermined adverse events;
in other words, the drugs and the combinations seem to be
as safe as expected. There is something like 30,000 women-years
of exposure to Arimidex, so we have a really good handle on
its safety profile. We already know about the pharmacokinetics
and pharmacodynamics of the interaction of anastrozole and
tamoxifen. We estimate that the predetermined number of events
for the formal analysis will be this summer, and we should
be ready to present the initial data at the end of the year.
—Michael
Baum, ChM, FRCS
| ATAC
Trial: Arimidex, Tamoxifen, Alone or in Combination
(closed to accrual) |
|
Eligibility |
Postmenopausal,
Stage 1 & 2 operable breast cancer, post-primary
treatment |
|
ARM 1 |
Arimidex
1 mg + placebo x 5 yrs |
|
ARM 2 |
Tamoxifen
20 mg + placebo x 5 yrs |
|
ARM 3 |
Arimidex
1 mg + tamoxifen 20 mg x 5 yrs |
|
|
ENDOMETRIAL
AND BONE SUBPROTOCOLS
Arimidex
lowers estradiol. It has no agonistic effect. I cannot think
of any plausible biological mechanism that it should have
any effect on the endometrium, other than perhaps making it
atrophic. So, it should either have a null effect or a protective
effect, and it will be interesting to compare the incidence
of endometrial cancer in the monotherapy arm and the combined
therapy arm, to see if one cancels out the other. The combination
might be very attractive, because, in theory, the effects
on breast cancer mortality should be synergistic. You can
make the same theoretical argument about bone — that
the agonist effect of tamoxifen might counteract an antagonistic
effect of anastrozole. The combination is rational and attractive
in theory, but we’ve had our fingers burned so many times
in the long history of clinical trials that the one thing
I hate to predict is the future.
—Michael
Baum, ChM, FRCS
ANASTROZOLE
VS TAMOXIFEN AS FIRST-LINE THERAPY OF METASTATIC DISEASE
| Trials
27 & 30: Phase III Randomized, Double-Blind
Study of Anastrozole vs Tamoxifen as First-Line
Therapy in Postmenopausal Women with Advanced Breast
Cancer (closed for accrual) |
|
Eligibility |
Postmenopausal,
locally advanced or metastatic breast cancer |
Bonneterre
J et al. Anastrozole versus tamoxifen as first-line
therapy for advanced breast cancer in 668 postmenopausal
women: Results of the tamoxifen or arimidex randomized
group efficacy and tolerability study. J
Clin Oncol 2000;18(22):3748-3757.
Abstract
Nabholtz
JM et al. Anastrozole is superior to tamoxifen
as first-line therapy for advanced breast cancer
in postmenopausal women: Results of a North American
multi-center randomized trial. J Clin
Oncol 2000;18(22):3758-3767.
Abstract
|
|
ARIMIDEX
VS TAMOXIFEN WITH RESPECT TO VAGINAL BLEEDING AND THROMBOEMBOLISM
(ADVANCED DISEASE)

No
differences were observed in weight gain, lethargy,
hot flashes, vaginal dryness, GI disturbance, depression
or tumor flare.
Buzdar
A. Anastrozole (Arimidex) versus tamoxifen as first-line
therapy for advanced breast cancer (ABC) in postmenopausal
(PM) women? Combined analysis from two identically designed
multi-center trials. Proc. ASCO 2000;
Abstract 609D
|
TRIALS
EVALUATING MORE THAN FIVE YEARS OF ENDOCRINE THERAPY
There’s
a lot of interest in defining the optimal duration of endocrine
treatment. The ATLAS trial is evaluating the fundamental question
of whether tamoxifen should be given for five or ten years.
There are also a number of other major trials looking at crossing
over from tamoxifen to an aromatase inhibitor at different
stages, but ATAC will be the only trial that will allow this
to be explored fully. One possibility is at five years to
re-randomize patients in ATAC to either stop treatment or
receive anastrozole or tamoxifen. It would be a three-way
randomization, independent of the first treatment. We can
consider this because the trial is so large — there are
more than 9,000 women, and we estimate that about 7,500 will
be free of disease at five years.
In terms
of continuing tamoxifen, for women who’ve had a hysterectomy,
the endometrial cancer risk is zero. If they also don’t
have risk factors for thromboembolic disease, perhaps tamoxifen
should be given for ten years? But primarily because of the
endometrial cancer and thromboembolic risk, the option of
swapping over to an aromatase inhibitor has excited a lot
of people. The thinking is that you may be able to continue
the endocrine protection against breast cancer by changing
the approach, and there’s a lot of research interest
in that concept. Certainly one of the things we found hardest
in the prevention trial was preparing women to stop taking
tamoxifen after five years, because they see it as sort of
a support, and it’s difficult to stop an intervention
when people perceive that it is doing them good.
—Jack
Cuzick, PhD
| ATLAS:
Phase III Study of Prolonged Adjuvant Tamoxifen
for Curatively Treated Breast Cancer.
Protocol |
|
Eligibility |
Curatively
treated breast cancer, currently on adjuvant
tamoxifen, any age, prior biologic treatment,
chemotherapy, XRT or surgery are allowed |
|
ARM 2 |
Continue tamoxifen 20 mg qd x 5 additional
years |
|
|
CROSSOVER
RESPONSES AFTER FIRST-LINE THERAPY OF
METASTASES (TRIALS 27,30)
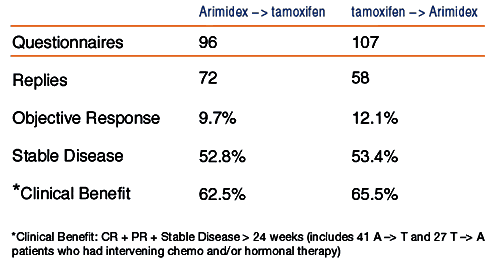
Robertson
J et al. European Journal of Cancer 2000;36(S5):
Abstract 219
|
SELECT PUBLICATIONS
| Baum
M. Use of aromatase inhibitors in the adjuvant treatment
of breast cancer. Endocrine-Related Cancer
1999;6(2):231-234.
Full-Text
Buzdar
AU. Role of aromatase inhibitors in advanced breast
cancer. Endocrine-Related Cancer 1999;6(2):219-225.
Full-Text
Buzdar
A. An overview of the use of non-steroidal aromatase
inhibitors in the treatment of breast cancer.
Eur J Cancer 2000;36 Suppl 4:S82-4.
Abstract
Casali
A et al. Letrozole for the treatment of pretreated
advanced breast cancer patients: Preliminary report.
J Exp Clin Cancer Res. 2000;19:17-9.
Abstract
Dowsett
M et al. The effect of anastrozole on the pharmacokinetics
of tamoxifen in postmenopausal women with early breast
cancer. Br J Cancer 1999;79(2):311-5.
Abstract
Dowsett
M et al. Effects of the aromatase inhibitor anastrozole
on serum oestrogens in Japanese and Caucasian women.
Cancer Chemother Pharmacol 2000;46(1):35-9.
Abstract
Goss
PE, Strasser K. Aromatase inhibitors in the treatment
and prevention of breast cancer. J Clin Oncol
2001;19:881-94.
Abstract
Kaufmann
M et al. Exemestane is superior to megestrol acetate
after tamoxifen failure in postmenopausal women with
advanced breast cancer: Results of a phase III randomized
double-blind trial. The Exemestane Study Group.
J Clin Oncol. 2000;18:1399-411.
Abstract
Lonning
PE. Pharmacology and clinical experience with exemestane.
Expert Opin Investig Drugs. 2000;9:1897-905.
Abstract
Lonning
PE et al. Activity of exemestane in metastatic breast
cancer after failure of nonsteroidal aromatase inhibitors:
A phase II trial. J Clin Oncol. 2000;18:2234-44.
Abstract
Osborne
CK. Aromatase inhibitors in relation to other forms
of endocrine therapy for breast cancer. Endocrine-Related
Cancer 1999;6(2):271-276.
Full-Text
Pritchard
KI. Current and future directions in medical therapy
for breast carcinoma: Endocrine treatment. Cancer
2000;88:3065-72.
Abstract
Robertson
JF et al. Static disease on anastrozole provides
similar benefit as objective response in patients with
advanced breast cancer. Breast Cancer Res Treat
1999;58(2):157-62.
Abstract
Vorobiof
DA et al. A randomized, open, parallel-group trial
to compare the endocrine effects of oral anastrozole
(Arimidex) with intramuscular formestane in postmenopausal
women with advanced breast cancer. Ann Oncol
1999;10(10):1219-25.
Abstract
Wolff
AC, Davidson NE. Primary systemic therapy in operable
breast cancer. J Clin Oncol 2000;18:1558-69.
Abstract
Yue
W et al. Aromatase within the breast. Endocrine-Related
Cancer 1999;6(2):157-164.
Full-Text
|
|
