Section 4
ER/PR Results and Endocrine
Therapy
DEFINING
"ER-POSITIVE"
The San
Antonio group looked at their very large database and found
that using IHC techniques, any receptor staining correlated
with a response to adjuvant endocrine therapy. So that’s
pretty much the definition we use. Having both estrogen and
progesterone receptors indicates a greater likelihood of response,
but we don’t use cutoffs like 10 or 20 percent of cells.
Traditionally, laboratory reports came back saying, “Minimal
staining not consistent with response to endocrine therapy.”
That’s not what we believe today.
—Monica
Morrow, MD
| Harvey
JM et al. Estrogen receptor status by immunohistochemistry
is superior to the ligand-binding assay for predicting
response to adjuvant endocrine therapy in breast cancer.
J Clin Oncol 1999;17:1474-81.
Abstract |
| 2000
NIH Consensus Development Conference on Early Breast Cancer,
Final Statement
Full-Text
The
decision whether to recommend adjuvant hormonal therapy
should be based on the presence of hormone receptors,
as assessed by immunohistochemical staining of breast
cancer tissue. If the available tissue is insufficient
to determine hormone receptor status, it should be considered
as being positive, particularly in postmenopausal women.
The small subset of women whose tumors lack estrogen
receptor protein but contain progesterone receptor also
appear to benefit from hormonal therapy... Adjuvant
hormonal therapy should be recommended to women whose
breast tumors contain hormone receptor protein, regardless
of age, menopausal status, involvement of axillary lymph
nodes or tumor size. While the likelihood of benefit
correlates with the amount of hormone receptor protein
in tumor cells, patients with any extent of hormone
receptor in their tumor cells may still benefit from
hormonal therapy. Such treatment has led to substantial
reductions in the likelihood of tumor recurrence, second
primary breast cancer and death persisting for at least
15 years of follow-up.
|
| FRACTION
OF PHYSICIANS WHO WOULD RECOMMEND ADJUVANT TAMOXIFEN |
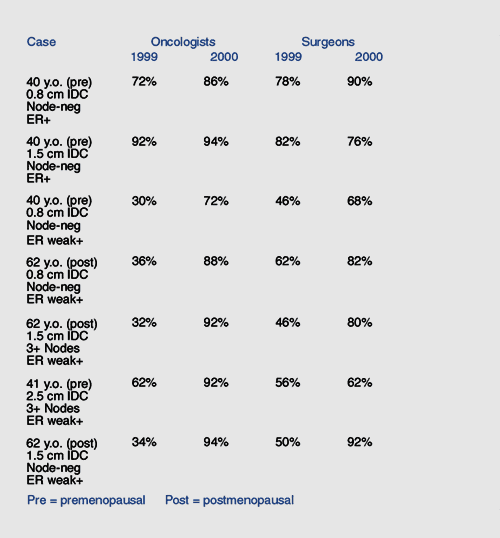

A
random series of telephone surveys was conducted in
June 1999 of 100 oncologists and 100 surgeons in the
United States. A second random survey was conducted
in June 2000 of different random samples of 100 oncologists
and 100 surgeons. Theoretical breast cancer case scenarios
were presented, and the physicians were asked how they
would manage such patients. The results show a marked
change in prescribing attitude during this time period
with a significantly greater fraction of physicians
indicating they would recommend adjuvant therapy with
tamoxifen in women with both receptor-positive cancers
and tumors with low but detectable estrogen receptors.
Motley
J. Recent Changes in Physicians' Interpretation
of Estrogen Receptor (ER) Assay Results: Adjuvant Endocrine
Therapy in Patients with Low, but Detectable ER.
Poster, 2001 Miami Breast Cancer Conference.
Full-Text
|
TAMOXIFEN
IN ER-NEGATIVE PATIENTS
The overview
data on that are not convincing one way or the other in terms
of contralateral breast cancer. The data are consistent with
either no effect in women with ER-negative tumors or an effect
the same size as for ER-positive tumors. There aren’t
many ER-negative women who have actually been given five years
of tamoxifen in trials. The evidence is sufficiently clear
now that tamoxifen is not good therapy for reducing recurrence
rates in ER-negative women, but we’re probably not going
to obtain much more data from the older adjuvant trials on
second cancers in ER-negative patients, because there aren’t
enough patients and events.
—Jack
Cuzick, PhD
Amongst
the unselected breast cancer patient population receiving
five years of adjuvant tamoxifen, there is a 45 percent reduction
in the risk of contralateral breast cancer. The problem is
that by the time we were doing trials of five years of tamoxifen,
they were all pretty much estrogen-positive breast cancers.
The fact that there’s a lack of evidence in ER-negatives
isn’t proving a negative. The risk of a contralateral
breast cancer in unselected women with breast cancer is 0.7
percent per year. So, that, in itself, makes those women at
higher risk than those entering the NSABP P-1 prevention trial.
One could argue that if you’re going to give tamoxifen
to women without breast cancer, you might give it to all women
with breast cancer irrespective of estrogen receptor status,
because they have a 3.5 percent chance of developing breast
cancer over five years.
—Michael Baum, ChM, FRCS
RELATIONSHIP
BETWEEN ER STATUS (BY IHC) AND OTHER PROGNOSTIC FACTORS

Adapted
from J Clin Oncol 1999;17:1474-81.
Abstract
|
“With
minimal training, pathologists in our laboratory were
in agreement on discriminating positive from negative
tumors in 99% of cases. The optimal cut point in our study
was a total IHC score of greater than 2, meaning that
even patients whose tumors scored 3 (corresponding to
as few as 1% to 10% weakly positive cells) had a significantly
improved response, compared with those who had lower scores...
...Many hospital and commercial laboratories have converted
to assessing ER status exclusively by IHC on archival
tissue. They use diverse methodologies, and most have
arbitrarily chosen 10% or even 20% positive tumor cells
as their cutoff for defining ER positivity, potentially
denying a substantial number of patients the benefits
of adjuvant hormone therapy.”
–Harvey et al. J Clin Oncol 1999;17:1474-81.
Abstract
|
SELECT
PUBLICATIONS
|
Elledge RM et al. Estrogen receptor (ER) and progesterone
receptor (PgR), by ligand-binding assay compared with
ER, PgR and pS2, by immuno-histochemistry in predicting
response to tamoxifen in metastatic breast cancer: A Southwest
Oncology Group Study. Int J Cancer 2000;89:111-7.
Abstract
Katzenellenbogen
BS et al. Molecular mechanisms of estrogen action:
Selective ligands and receptor pharmacology. J
Steroid Biochem Mol Biol 2000;74:279-85.
Abstract
Katzenellenbogen
BS et al. Estrogen receptors: Selective ligands,
partners and distinctive pharmacology. Recent
Prog Horm Res 2000;55:163-93; discussion 194-5.
Abstract
Rhodes
A et al. Study of interlaboratory reliability and
reproducibility of estrogen and progesterone receptor
assays in Europe. Documentation of poor reliability
and identification of insufficient microwave antigen
retrieval time as a major contributory element of unreliable
assays. Am J Clin Pathol 2001;115(1):44-58.
Full-Text
|
|
