Section 3
Ductal Carcinoma In Situ
NSABP
PROPOSED TRIAL COMPARING ARIMIDEX® (anastrozole) TO TAMOXIFEN
IN DCIS
The driving
force of current research is to move away from the concept
that DCIS is simply a surgical problem — and that if
you obtain 10 mm margins, the patient is cured and no adjuvant
therapy is needed. And it’s not really important to argue
about whether there’s a set of patients who don’t
need radiation therapy.
Even if
we take out the index DCIS, the risk for these women to have
another tumor in either breast in the future is at least as
high or higher than the risk for women in the NSABP P-1 prevention
trial. So, chemoprevention in DCIS is an important issue,
and we need to find out how to do this best.
Nobody
believed when we started with tamoxifen that it would be a
"home run" and end all inquiry. Even with the prevention trial,
as enormously successful as it was in reducing the incidence
of cancer by 50 percent, everybody understands that there
must be a more effective or safer drug.
The ATAC
trial, which has past accrual and is nearing analysis, will
answer the question about anastrozole in invasive breast cancer.
We need to ask the same question in non-invasive disease.
We are focusing on Arimidex, because it has been well-tested,
and there are the two studies in advanced disease that show
that it’s equal or superior to tamoxifen.
—Richard
Margolese, MD
| Proposed
NSABP DCIS Trial : Tamoxifen versus Arimidex in
Post menopausal Patients with Ductal Carcinoma In
Situ |
|
Eligibility |
Postmenopausal, DCIS, treated with lumpectomy
& XRT |
|
ARM 1 |
Tamoxifen
20 mg qd x 5 yrs |
|
ARM 2 |
Arimidex
1 mg qd x 5 yrs |
Margolese R. Rationale for proposed National
Surgical Adjuvant Breast and Bowel Project (NSABP):
DCIS Trial. Tamoxifen versus Arimidex® (anastrozole)
in postmenopausal patients with Ductal Carcinome
In Situ. Poster, 2001 Miami Breast Cancer
Conference.
Full-Text
|
|
KEY
PRIOR NSABP DCIS TRIALS
| NSABP
B-17: Phase III Randomized Study of Postoperative
Radiotherapy Following Segmental Mastectomy and
Axillary Dissection in Patients with Noninvasive
Intraductal Adenocarcinoma of the Breast (closed
to accrual)
Protocol |
|
Eligibility |
Small, localized DCIS, treated with lumpectomy
(- margins) |
|
ARM 1 |
No
further treatment |
Fisher B et al. Lumpectomy compared with lumpectomy
and radiation therapy for the treatment of intraductal
breast cancer. N Engl J Med
1993; 328:1581-1586.
Abstract
|
|
| NSABP
B-24: Phase III Randomized Trial of Adjuvant Tamoxifen
vs Placebo Following Breast Irradiation in Patients
Who Have Undergone Lumpectomy for Noninvasive Intraductal
Carcinoma (DCIS) of the Breast (closed to accrual)
Protocol |
|
Eligibility |
DCIS (local or diffuse), treated with lumpectomy
(+/- margins) & XRT |
|
ARM 2 |
Tamoxifen 20 mg qd x 5 yrs |
Fisher B et al. Tamoxifen in treatment of intraductal
breast cancer: National Surgical Adjuvant Breast
and Bowel Project B-24 randomised controlled trial.
Lancet 1999;353(9169):1993-2000.
Abstract
|
|
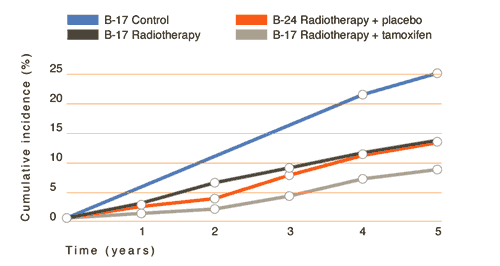
Modified from Lancet 1999;353(9169):1993-2000.
Abstract |
NSABP
trials of patients receiving lumpectomy for DCIS. Cumulative
incidence of all invasive and noninvasive events in the ipsilateral
and contralateral breast in NSABP B-17 and B-24 studies. Note
the stepwise improvements in outcome with the addition of
XRT and tamoxifen to lumpectomy for DCIS.
KEY
CURRENT DCIS TRIAL
| RTOG-9804;
RTOG-DEV-1026: Phase III Randomized Study of Tamoxifen
with or without Radiotherapy in Women with Ductal
Carcinoma In Situ (DCIS) of the Breast
Protocol |
|
Eligibility |
DCIS,
no prior chemo- or XRT or concurrent hormone
treatment |
|
ARM 1 |
Tamoxifen x 5 years |
|
ARM 2 |
Radiotherapy daily 5 times per week for 5.5
weeks + tamoxifen x 5 years |
|
|
ADJUVANT
THERAPY FOR DCIS PATIENTS WITH POSITIVE SENTINEL NODES
This is
an extremely challenging issue. There clearly are patients
with DCIS diagnosed by core biopsy techniques who have invasive
cancer in their final specimens. Once they have invasive cancer,
I don’t consider them in the DCIS pool. So the first
step when a "DCIS patient" has a positive node by H &
E is to go back and ask your pathologist to make extra sections
and look very hard for invasive carcinoma. The people from
Moffitt have looked retrospectively at DCIS patients with
IHC-positive nodes and found no survival impact, but that
study didn’t have much statistical power. We have no
reliable data to guide us in that situation, which is the
reason I don’t do IHC in routine practice.
—Monica
Morrow, MD
NATURAL
HISTORY
DCIS is
over-diagnosed — certainly in premenopausal women. It
is likely that only one in five, if undetected, would progress
to become invasive breast cancer. But having detected it,
we then have to treat it, and I think one of the most dishonest
things about promoting mammographic screening for women under
50 is the idea of "Come for screening. We’ll save your
life and save your breast." But your breast is not saved,
because DCIS is often outside one quadrant, so you have this
extraordinary paradox that women think DCIS is early breast
cancer. And yet, in the U.K. and the U.S.A., about 40 percent
of premenopausal women with DCIS end up having a mastectomy.
Whereas, if it was left to appear as an invasive breast cancer,
the treatment would be a lumpectomy. No one can explain that
mismatch.
—Michael
Baum, ChM, FRCS
SELECT
PUBLICATIONS
|
Ernster VL et al. Mortality among women with ductal
carcinoma in situ of the breast in the population-based
Surveillance, Epidemiology and End Results program.
Arch Intern Med 2000;160:953-8.
Abstract
Pendas
S et al. Sentinel node biopsy in ductal carcinoma
in situ patients. Ann Surg Oncol 2000;7:15-20.
Abstract
Silverstein
MJ. Ductal carcinoma in situ of the breast.
Annu Rev Med 2000;51:17-32.
Abstract
Winchester
DP et al. The diagnosis and management of ductal
carcinoma in situ of the breast. CA Cancer J
Clin 2000;50:184-200.
Full-Text
|
OTHER
RESOURCES
Silverstein
MJ (Editor). Ductal Carcinoma In Situ of the Breast.
Baltimore: Williams & Wilkins, 1997.
Web link
Comprehensive reference with 114 contributors covering basic
science and clinical aspects of DCIS.
Current
Problems In Cancer. 2000;24(3).
Web link
Issue covering epidemiology, medical imaging, pathobiology,
surgery and adjuvant treatment of DCIS.
|
