| You are here: Home: BCU 2|2002: Michael Baum, ChM, FRCS

Edited Comments by Dr Baum
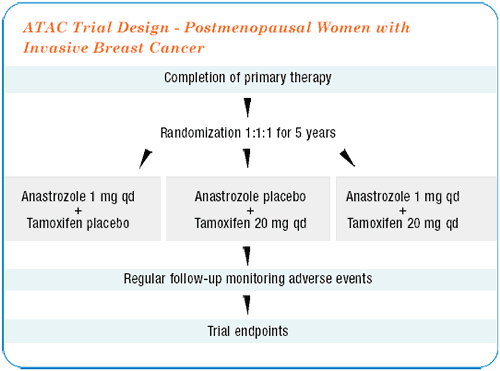
SUMMARY OF ATAC RESULTS
There are over 9,000 patients in this study from all over the world, with just over 3,000 patients in each arm. On average the patients were exposed to two and a half years of the treatment. There was a statistically predetermined number of events we were looking for, which triggered the first formal analysis.
The headline news is that it looks as if there is something after tamoxifen — there is a significant advantage to anastrozole compared with tamoxifen. The real surprise is that the combination of anastrozole and tamoxifen looks no different than tamoxifen alone.
What makes these early ATAC results even more extraordinary is that about 15% of the trial population was ER-negative or ERunknown. When you look at an analysis of the subgroup of known ER-positive patients in the study, the effect comes out even stronger. The hazard ratio for anastrozole versus tamoxifen is 0.78 — equivalent to a 22% relative reduction in risk for recurrence compared to tamoxifen.
| Anastrozole’s Profile |
 |
- Highly selective, potent aromatase inhibitor
- Nonsteroidal
- Orally active (1 mg)
- Once-daily dosing
|
- Superior to tamoxifen in postmenopausal women with estrogen receptor-positive advanced breast cancer
- Survival advantage vs. megestrol acetate in metastatic disease
- Over 460,000 patient-years experience
|
|
REDUCTION IN CONTRALATERAL BREAST CANCERS
Tamoxifen produces about a 50% reduction in contralateral breast cancers, and in this trial, anastrozole produced a staggering 58% reduction over tamoxifen — and the difference emerged within one year. If these findings hold up, we can add another 60% reduction on top of the 50%, and really start translating that into effective chemoprevention of breast cancer.
| Subprotocols of the ATAC Trial |
 |
- Pharmacodynamic and pharmacokinetic profiles
- Modulation of lipoprotein profiles
- Endometrial status
- Bone mineral metabolism
- Quality of life
|
SAFETY PROFILE OF ANASTROZOLE
One of the most exciting parts of the ATAC trial is the safety profile of anastrozole. There was a highly significant reduction in the incidence of hot flashes, vaginal discharge and vaginal bleeding. This reduction in vaginal bleeding is significant, because this will cut down the number of women referred to gynecologists to exclude endometrial cancer.
| ATAC Trial - Study Endpoints |
| Primary Endpoints
- Disease-free survival
- Locoregional or distant recurrence, new primary breast cancer, or death from any cause
- Safety/Tolerability
|
 |
| Secondary Endpoints
- Incidence of new breast (contralateral) primaries
- Time to distant recurrence
- Survival (data will be mature in » 2 years)
- Hormone receptor-positive population (protocol-defined sub-group)
|
Perhaps even more important is the significant reduction in the anastrozole arm in life-threatening events such as strokes, cerebrovascular accidents and thromboembolic events.
In terms of side effects, about 8% of women complain about arthralgias. There is also a numerically modest (about 4%) but highly significant excess fracture rate in the anastrozole arm. Apart from bone mineral density — which I think we can handle if we anticipate it — the safety profile strongly favors anastrozole over tamoxifen.
potential role of bisphosphonates combined with anastrozole in the adjuvant setting
I’m convinced that adjuvant bisphosphonates reduce the risk of bone metastases during therapy. We need to determine if there is synergism between aromatase inhibitors and bisphosphonates. We are discussing the possibility of a second randomization within the ATAC trial to anastrozole alone versus anastrozole plus a bisphosphonate.
| ATAC Trial - Patient Characteristics* |
 |
- Mean age in the anastrozole arm was 64.1 years
- 83.7% of patients in the anastrozole arm were ER-positive
- 47.8% of patients in the anastrozole arm were treated with mastectomy
- 22.3% of patients in the anastrozole arm were treated with chemotherapy
*patients in all arms were similar
Derived from a presentation by Michael Baum, 2001 Annual San Antonio
Breast Cancer Symposium |
| Summary of ATAC Trial Outcomes |
 |
| 9,366 evaluable patients
- At a median treatment duration of 2.5 years, anastrozole demonstrated superior efficacy and tolerability compared to tamoxifen
- Anastrozole was superior to tamoxifen in terms of disease-free survival in the
overall population (relative reduction of 17%) and in estrogen receptor-positive patients (relative reduction of 22%)
- Anastrozole was superior to tamoxifen in terms of the incidence of
contralateral breast cancer in the overall population (relative reduction of 58%)
- There were 156 patients with distant metastases in the anastrozole arm and
181 in the tamoxifen arm (not statistically different)
- There were only a total of five breast cancer deaths in the three treatment arms
|
 |
| Anastrozole was better tolerated than tamoxifen with respect to:
- Endometrial cancer
- Vaginal bleeding
- Vaginal discharge
- Ischaemic cerebrovascular events
- Venous thromboembolic events
- Hot flashes
- Weight gain
|
 |
| Tamoxifen was better tolerated than anastrozole with respect to:
- Musculoskeletal disorders (arthralgias)
- Fractures
|
 |
| Derived from a presentation by Michael Baum, 2001 Annual San Antonio Breast Cancer Symposium
Baum M. The ATAC (Arimidex, Tamoxifen, Alone or in Combination) adjuvant breast cancer trial in postmenopausal (PM) women. Breast Cancer Res Treat 2001; 69 (3): Abstract 8. |
adjuvant endocrine treatment: implications atac
In the evolution of science and medicine, there are periods of uncertainty, and we are living in such a time right now related to these ATAC findings. If the efficacy advantage for anastrozole continues to be seen, then we can start making therapeutic recommendations, but presently we have only two and one-half years of treatment data. We cannot be certain about what will happen with further therapy.
Newly diagnosed women should be informed of the ATAC data in a responsible way, and most of them will make a rational decision. Tamoxifen should continue to be considered the gold standard, at least until the trial results are updated this May at ASCO 2002. However, anastrozole is a legitimate nonprotocol adjuvant option where there are contraindications to tamoxifen. If women are already on adjuvant tamoxifen, it would be hazardous to switch them to anastrozole, since we haven’t tested that therapeutic approach.
using other aromatase inhibitors in the adjuvant setting
I do not know if this is a class effect of aromatase inhibitors. I can only speak for anastrozole in the ATAC trial. There are subtle differences in pharmacology and pharmacokinetics between the two nonsteroidal aromatase inhibitors (anastrozole and letrozole) and even more with the steroidal aromatase inhibitor, exemestane. These differences could lead to different results. Although I suspect that they will have similar efficacy, I don’t think we can assume they will have the same trade-off in adverse events.
aromatase inhibitors in the neoadjuvant setting
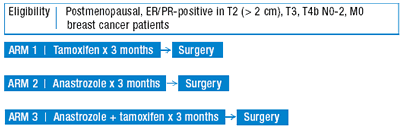
The IMPACT trial — currently being conducted at the Royal Marsden Hospital — is similar to ATAC but is in the preoperative setting. The added advantage of this type of study is the ability to take biological samples of the primary tumor in the preoperative phase to study what happens at the cellular level in the face of aromatase inhibitors.
| IMPACT TRIAL: A Randomized Double Blind Trial of Preoperative Tamoxifen, Anastrozole or the Combination in Postmenopausal Breast Cancer Patients Open Protocol |
|
| 
Study Contact
Ian Smith, MD, Chair
Royal Marsden Hospital
London, United Kingdom |
|
Anastrozole as Neoadjuvant Therapy in Hormone-Dependent
Locally Advanced [Stage IIIA (n=29) and IIIB (n=45)]
Postmenopausal Patients |
| |
Neoadjuvant anastrozole
(n=74) |
Objective Response (PR + CR)
|
61 (83%) |
Partial Response (PR)
|
42 (57%) |
Complete Respone (CR)
|
19 (26%) |
Pathological Complete Response
|
14 (23%) |
Pathological Partial Response
|
47 (64%) |
| No Response |
13 (18%) |
| Derived from Milla-Santos A et al. Breast Cancer Res Treat 2001; Abstract 302. |
the value of screening mammography
I don’t think mammographic screening has anywhere to go. Also, as systemic adjuvant treatments improve, there will be less potential impact of screening. Even in the “bad old days” before successful adjuvant therapy, screening led to a 25% reduction in mortality, which translates into saving one in a thousand lives with ten years of screening.
In addition, you cannot compare the quality of current treatment trials (like ATAC) with the quality of the older mammographic screening trials. As a clinical trialist, if I had attempted to submit an abstract on a trial of adjuvant therapy designed like most of the screening trials, it would not have been approved. Yet we have accepted shabby clinical trials to demonstrate the benefits of mammographic screening.
Select Publications
|
