| You are here: Home: BCU 2|2002: Robert B Livinnston, MD

Edited Comments by Dr Livingston
overview of proposed phase III adjuvant intergroup study
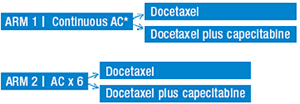
We are planning a Phase III, randomized, adjuvant trial in women with hormone receptor-negative, node-positive or high-risk, nodenegative disease. This study should be open to accrual in about a year. The trial is designed to compare two different schedules of AC followed by either docetaxel alone or in combination with capecitabine.
Continuous — or metronomic — chemotherapy may be more effective than standard intermittent chemotherapy, because it results in a greater degree of antiangiogenic and antitumor activity. The initial randomization of this trial will be “continuous AC” versus six cycles of AC. There’s a growing opinion — at least in this country — that four cycles of AC is probably an inadequate duration of treatment.
It was the opinion of the other Intergroup chairs, as well as myself, that we should give the same duration of treatment in both arms. So, the patients in the second arm will receive six cycles of AC.
The second randomization is based on the phase III randomized trial conducted by Joyce O’Shaughnessy, in which women with anthracycline-refractory stage IV disease received docetaxel or a lower dose of docetaxel plus capecitabine. The second randomization in this study will be to either docetaxel alone or docetaxel plus capecitabine.
| Proposed Intergroup Trial: Phase III Study of Adjuvant Continuous versus Standard Intermittent Doxorubicin and Cyclophosphamide Followed by Capecitabine and Docetaxel versus Docetaxel Alone in Hormone Receptor- negative, Node-positive or High-risk, Node-negative Breast Cancer
Note: This trial is expected to begin accrual within the next year. Details of the trial are subject to change. |
|
Protocol ID
*Continuous AC = doxorubicin q week; cyclophosphamide q day (orally)
Principal Investigator:
G Thomas Budd, M D
Cleveland Clinic Foundation |
|
capecitabine/docetaxel combination in clinical practice
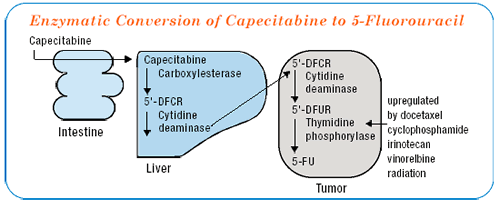
Outside the context of a clinical trial, we are using the combination of capecitabine and docetaxel, primarily in women who have had previous anthracycline treatment but were not exposed to either a fluorinated pyrimidine or taxane. We would start at a lower dose, because in Joyce O’Shaughnessy’s phase III trial, a 25% dose reduction for both docetaxel and capecitabine was necessary in most women. They were then able to tolerate that combination at that dose for the remainder of their treatment.
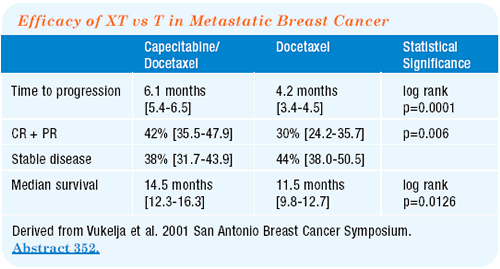
| Phase III Trial of Docetaxel-Capecitabine (XT) Combination Therapy vs Docetaxel
Monotherapy (T) in Metastatic Breast Cancer Closed Protocol |
|
|
X = capecitabine, T = docetaxel |
|
| XT versus T: Baseline Characteristics |
- Age (median=52-years-old), performance status, hormone - receptor status and sites of metastases were equivalent between groups
- Two - thirds of patients had >= 3 metastatic sites
- Prior chemotherapies: 100% had anthracyclines, 90% had alkylating agents, 75% had 5-FU and about 10% had paclitaxel
- No difference in percentage of patients being treated first-line and about two - thirds received XT or T as second- or third-line treatment
|
 |
| XT versus T: Dose Reductions and Quality of Life
- Dose reductions to 75% of initial dose were observed in two - thirds of patients in the XT arm for either capecitabine or docetaxel and in about one-third of docetaxel only patients
- Quality of life — assessed by EORTC QLQ-C30 global health status — showed a trend favoring XT compared to T
Derived from Vukelja S et al. Breast Cancer Res Treat 2001; Abstract 352. |
| XT versus T: Post-study Treatment |
| Approximately two - thirds of patients received chemotherapy after XT or T.
17% of patients in the docetaxel only arm subsequently received capecitabine. |
 |
| Derived from Miles et al. Breast Cancer Res Treat 2001; Abstract 442
Vukelja S et al. Breast Cancer Res Treat 2001; Abstract 352. |
use of adjuvant taxanes in er-positive, her2-negative patients
If a woman is ER-positive, HER2-negative and has fewer than four positive nodes, then I would use CMF followed by tamoxifen. I wouldn’t use taxanes or doxorubicin. No trials have demonstrated that for these “garden-variety” patients, anthracycline-based therapies are any better, and neither the CALGB nor the NSABP have shown an advantage for the subsequent administration of a taxane after initial AC treatment in ER-positive women.
treatment of node-positive, her2-positive patients
In women who are node-positive and HER2-positive, we routinely use anthracycline-based therapy and taxane-based consolidation, regardless of their hormone receptor status. There is a reasonable response rate to taxanes in HER2-positive disease, and it’s probably the same for HER2-negative disease that has become anthracyclinerefractory. We do not have data from any completed randomized trial that speaks to the value of a taxane in ER-positive patients.
We treat women who are ER-positive and HER2-positive differently than those who are HER2-negative. We believe that HER2 overexpression confers a much greater probability of resistance, even to anthracycline-based combinations, and certainly there is a greater probability of resistance to hormonal therapies, namely tamoxifen.
trials of antitubulin combinations
Our group has been very interested in antitubulin combinations — primarily taxanes and vincas. Since the most useful vinca for the treatment of breast cancer is vinorelbine, our trials involve that drug. In the setting of anthracycline-refractory stage IV breast cancer, we have done a series of studies using a dose-dense and dose-intense approach to the administration of vinorelbine, both alone and in combination with taxanes.
management of er-negative, metastatic breast cancer
A woman with ER-negative metastatic breast cancer should be offered an anthracycline-based regimen if she has not already received it in the adjuvant setting or if she recurs over one year since receiving an adjuvant anthracycline.
We do not have irrefutable data that women with ER-negative, HER2-negative disease do better with an anthracycline-based regimen, but I would be very hesitant to give them nonanthracycline-based therapy. In a nonprotocol setting, the combination of doxorubicin and docetaxel would be reasonable, although I also use capecitabine and docetaxel.
There are some women who would be better candidates for singleagent capecitabine. One must individualize therapy based on what you see in that particular woman. For a woman in whom I was concerned about myelosuppression, or if the patient already had neurotoxicity, I would favor single-agent capecitabine.
treatment algorithm for metastatic breast cancer
There are several algorithms for the management of a typical case of newly diagnosed metastatic breast cancer, and a well-informed, rational medical oncologist can make a good case for any of them. My approach is influenced by my interest in evaluating continuous drug exposure and combined antitubulin therapies. Empirical evidence based on phase II studies demonstrates that continuous chemotherapy with the AC regimen and combined antitubulins may be better.
One could take either of those regimens and potentially add a drug like capecitabine. Combining capecitabine with continuous AC may be problematic, but you may be able to add it to a taxane/vinorelbine regimen.
combination versus single-agent chemotherapy
Many young women with visceral-dominant disease, who have failed initial treatment, want an aggressive approach. If you achieve an objective response without significant toxicity in a young and relatively healthy woman, that individual’s quality of life is likely to improve. However, you are more likely to achieve an objective response with combination chemotherapy. In postmenopausal women with a single site of disease, I would usually use a sequential single-agent approach.
treatment of women who received prior adjuvant act
In an off-study situation, we combine vinorelbine with weekly taxane administration. There is reasonably good evidence that 20 to 30% of metastatic breast cancer patients with prior exposure to every-three-week taxane treatment will achieve an objective response when subsequently given either paclitaxel or docetaxel on a weekly schedule. So, our rationale is we can give vinorelbine at almost full dose and combine it with a taxane and get both drugs in at reasonably close to full dose.
treatment of metastatic disease in her2-positive women
At our institution, women with HER2-positive metastatic breast cancer receive trastuzumab plus an antitubulin combination, which does not appear to be associated with any unusual or unexpected toxicities.
In a nonprotocol setting, trastuzumab plus either vinorelbine or paclitaxel are very reasonable regimens. Only if a woman is not a candidate for chemotherapy or refuses chemotherapy, do we use single-agent trastuzumab. There is usually some rational combination to offer.
duration of trastuzumab therapy
Trastuzumab should be continued at least until progression, and sometimes we continue it beyond that point. There is no answer about when to discontinue trastuzumab. Only a randomized trial will answer that question.
We are no longer in a situation where there is certainty that the continuation of trastuzumab is fruitless. If one believes trastuzumab is working in part through the potentiation of some other mechanism, then you must open your mind to the possibility that trastuzumab may potentiate vinorelbine after paclitaxel, for example. I typically treat a woman with a chemotherapeutic regimen plus trastuzumab until I see a maximum response or she has serious toxicity problems. Then, I discontinue the chemotherapy and continue the trastuzumab. Since there are no randomized trials to guide us, this is an individual practice decision.
treatment of metastatic breast cancer in er-positive, her2-negative women
In ER-positive, HER2-negative women without visceral-dominant disease, we initially treat with hormone therapy alone. If they have bone metastases, we use hormone therapy plus a bisphosphonate. In women with visceral disease, liver involvement predicts a lower likelihood or a shorter duration of response to hormone therapy. The same is not true of lung, bone, skin or chest wall involvement. If a woman with visceral disease is not a good candidate for hormone therapy alone, I use hormonal therapy plus chemotherapy, which is typically CMF.
pet scanning with labeled estrogens
David Mankoff at the University of Washington is conducting an experimental study evaluating PET scanning with labeled estrogens. The concept is very similar to the glucose PET scan. Basically, you positron label an estradiol molecule and inject it into the patient. Only those sites with the ability to selectively concentrate estradiol (i.e., those with an active estrogen receptor) take it up in sufficient quantity in order to see a hot spot in terms of PET emissions. To date, women with evidence of facilitated estrogen uptake into their tumor sites are the ones responding to hormone therapy.
Select Publications
|
