| You are here: Home: BCU 2|2002: Mark Pegram, MD

Edited Comments by Dr Pegram
overview of her2 biology
There are four members of the human epidermal growth factor receptor (HER) family — HER1, HER2, HER3, HER4. These receptors interact with and provide signals to cells concerning their biologic behavior. Unlike HER2, the other receptors (HER1, HER3 and HER4) have ligands that bind to them directly.
The most probable explanation for HER2’s lack of direct ligand binding is that HER2 is the “driver” of signaling for all members of the HER family. For example, ligands bind to HER1, but rely on HER2 to transmit and amplify their signal.
The HER family is analogous to a stereo system with a compact disk, tape and DVD player — each playing different types of media. The CDs, tapes, and DVDs are like ligands. The cell can “listen” to several types of ligands, but central to the function of the stereo system is the amplifier. HER2 is equivalent to the amplifier. When the HER2 receptor is overexpressed, the “volume” is turned all the way up on the stereo. This causes breast cancer cells to proliferate rapidly.
| Molecular Targets for Determining HER2 Status and the Tests Used to Detect These Molecules.
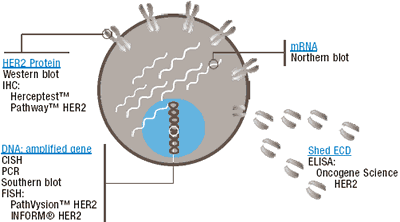
Adapted from Schaller G et al. Ann Oncol 2001;12:(Suppl 1):S97-S100
mRNA = messenger RNA
ECD = ext racellular domain
ELISA = enzyme-linked immunosorbent assay
IHC = immunohistochemistry
|
CISH = chromogenic in situ hybridization
PCR = polymerase chain reaction
FISH = fluorescence in situ hybridization |
|
|
mechanism of antitumor action of trastuzumab
Normal breast tissue has approximately 20,000 HER2 receptors per cell. In contrast, breast cancer cells with HER2 gene amplification have about two million receptors per cell. The high density of these receptors causes cell proliferation. This same high density of HER2, however, enhances antibody binding, making it a perfect target for trastuzumab.
Trastuzumab binds to the HER2 receptor, covering the breast cancer cell with antibodies just like antibodies cover bacteria when they fight off an infection. This antibody binding creates a potent signal for the immune system to attack the breast cancer cells. A higher density of HER2 leads to increased antibody binding to the cells and a greater immune response. Perhaps equally important, antibody binding also disrupts HER2’s signaling function.
her2 gene amplification in breast cancer cells
Approximately 20% of women with breast cancers have HER2 gene amplification. Dr Giovanni Pauletti, together with Dr Dennis Slamon at our institution, studied a large cohort of more than 900 primary breast cancer patients from South Australia. In these tumor samples, the HER2 gene amplification rate using fluorescence in situ hybridization (FISH) was 20%. This 20% rate was also confirmed by Dr Mike Press at USC, our collaborator in the Breast Cancer International Research Group (BCIRG), in the first 600 samples collected for the BCIRG’s adjuvant trastuzumab study.
evaluation of her2 assays
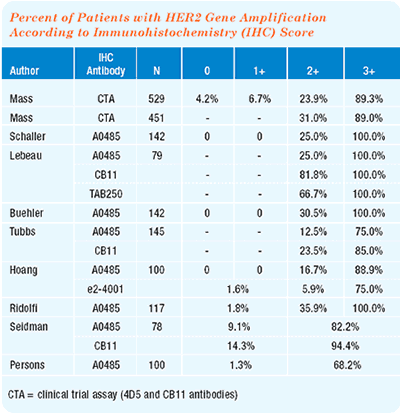
There is no question that FISH will replace immunohistochemistry (IHC) — sooner rather than later. FISH is clearly more accurate. Accuracy is the only acceptable way to go in medical diagnostics when dealing with patients’ lives.
The inappropriate use of trastuzumab in women who will not benefit (i.e., those with false-positive IHC results) is equally as bad as denying trastuzumab to women with false-negative IHC results. One FISH assay is less expensive than one dose of trastuzumab. From an economic perspective, FISH is more cost-effective.
FISH is widely available. It may need to be sent to a reference laboratory, but almost every test that you have sent to your local hospital laboratory gets sent out. So, they’ll just send the FISH slides in the same box, and you’ll get the result in the mail in a day or two. It’s trivial.
Not every patient who already has an IHC result must have a FISH analysis. It comes down to clinical judgment. If the clinical history fits the IHC result, then you’re probably safe with an IHC assay. In a patient without a prior HER2 assay, I would perform a FISH analysis first.
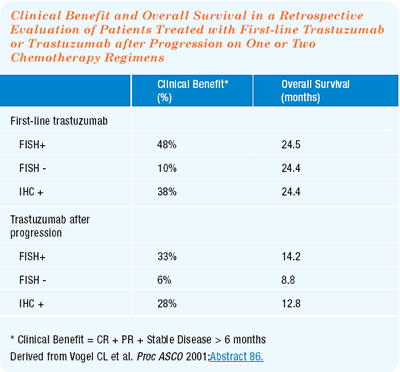
single-agent trastuzumab
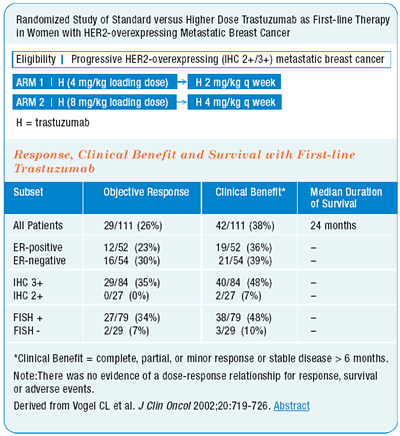
I am impressed with Chuck Vogel’s data on single-agent trastuzumab. The survival data with single-agent trastuzumab looks almost identical to that reported in the chemotherapy plus trastuzumab pivotal trial. Additionally, the patient demographics were surprisingly similar. These are not randomized trials, and I admit that I am making dangerous cross-trial comparisons. But, I am impressed that there may be a survival benefit to trastuzumabbased treatment in HER2-positive patients.
management of her2-positive patients
The management of HER2-positive, ER-negative women with metastases should take into consideration disease burden, patient age and prior therapy. If the woman has a high disease burden, a young age and a good performance status, then I would use trastuzumab in combination with chemotherapy.
In elderly women with smaller volume disease, who are not good candidates for chemotherapy, single-agent trastuzumab results in a good clinical response in about one-third of patients. Including patients with disease stabilization of six months or more, response rates are even higher. About half of the patients derive some meaningful clinical benefit from single-agent trastuzumab, without the side effects associated with chemotherapy.
trastuzumab scheduling
We, like many others, have been compelled to switch to triple-dose trastuzumab administered every three weeks. When we discuss Dr Brian Leyland-Jones’ results from his pharmacokinetic studies with the triple-dose, every-three-week schedule with our patients, many opt for that schedule. So far, we have not had any problems with that schedule.
At this point, however, we really do not have comparative data from large randomized trials. Many of the cooperative group studies evaluating trastuzumab are adopting the every-three-week, triple-dose schedule. In the BCIRG adjuvant trastuzumab trial, trastuzumab will be given following chemotherapy on an every- three- week schedule. Over the next couple of years, hundreds of patients will be treated with the every-three-week schedule and safety data will be collected.
From a theoretical point of view, I am not concerned about efficacy. The peak trastuzumab blood levels are actually higher on the every- three- week schedule. Since there is actually more trastuzumab on board, if anything, there could be greater efficacy. I do not believe that will necessarily be the case, but certainly there is no theoretical reason to expect a decrease in efficacy.
duration of trastuzumab treatment
No clinical information exists to guide the decision about continuing treatment with trastuzumab. In metastatic breast cancer patients, there comes a point where medical therapy is no longer effective. If the disease is end-stage, we certainly stop treatments. In women with a good performance status and no side effects, we continue trastuzumab. In the absence of data, we have been hesitant to discontinue it. Although trastuzumab may not prevent progression, it may slow it down. We do not have clinical answers yet.
hormonal therapy in combination with trastuzumab
There is scientific rationale to combine hormonal therapy with trastuzumab. There is cross-talk between the HER family signal pathway and the estrogen receptor. The estrogen receptor is downregulated by HER2 signaling; therefore, blocking HER2 may restore hormone sensitivity through the mitigation of this estrogen receptor downregulation phenomenon.
In animal models, Dr Rich Pietras at UCLA has studied the combinations of tamoxifen and trastuzumab as well as fulvestrant and trastuzumab. He has demonstrated striking beneficial results. There is also an ongoing study with anastrozole and trastuzumab. In ER-positive women receiving tamoxifen or an aromatase inhibitor in combination with trastuzumab, I have observed clinical responses. Prior to the availability of trastuzumab, I did — on occasion — observe HER2-positive women respond to hormonal therapy. In the right clinical scenario, it is reasonable to consider hormonal agents in combination with trastuzumab.
In ER-positive, HER2-positive women, I usually use trastuzumab with or without hormonal therapy, and I almost always use hormonal therapy because of its low incidence of side effects. In women with ER-positive, HER2-positive disease not enrolled in a trial, it is a very logical combination that I consider early on.
It would be reasonable to give women with low-volume, ER-positive disease a trial of hormonal therapy alone. In those women without a rapid response, I would move on quickly to trastuzumab-based therapy for those who are HER2-positive.
trastuzumab in the adjuvant setting
This is not the standard of care; therefore, we do not use trastuzumab routinely as adjuvant therapy. We first look for a study that the patient might enter, and we are fortunate to be able to have that as our reflex, so we don’t have to make the difficult decisions. There is a good chance that the trastuzumab adjuvant trials will eventually be positive. Historically, most therapies that prolong survival in metastatic breast cancer tend to have even greater benefits in the adjuvant setting.
bcirg adjuvant trastuzumab study
The Breast Cancer International Research Group’s (BCIRG’s) trastuzumab adjuvant trial 006 is evaluating conventional chemotherapy strategies — four cycles of AC followed by four cycles of docetaxel — in combination with trastuzumab. The arm we are most excited about is the docetaxel-carboplatin-trastuzumab arm.
This is a nonanthracycline combination of synergistic drugs. There is synergy between docetaxel-trastuzumab as well as carboplatintrastuzumab. In addition, we don’t have to worry as much about cardiotoxicity with that combination.
Trastuzumab Interactions with Chemotherapy Agents:
Rationale for Current Clinical Trials |
| Cisplatin |
synergism
|
| Thiotepa |
synergism |
Etoposide
|
synergism |
| Doxorubicin |
addition |
| Paclitaxel |
addition |
| Methotrexate |
addition |
| Vinblastine |
addition |
| 5 - fluorouracil |
antagonism |
| Derived from Pegram M et al. Oncogene 1999;18:2241-2251. |
|
| It is now generally accepted that identification of molecular alterations which play a role in the pathogenesis of specific human malignancies will lead to the development of targeted therapeutics which should be more effective and less toxic than currently available agents...
Studies leading to a greater understanding of the biological consequences of HER2/neu-directed therapies should allow the integration of this molecularly-targeted approach with currently available cancer treatments. The additive or synergistic therapeutic interaction between rhuMAb HER2 and a number of chemotherapeutic drugs suggests that such combinations could be successfully exploited in future human clinical trials.
—Pegram M et al. Oncogene 1999;18:2241-2251. |
| Phase III Randomized Study of Adjuvant Doxorubicin, Cyclophosphamide and Docetaxel with or without Trastuzumab (Herceptin) versus Trastuzumab, Docetaxel and Either Carboplatin or Cisplatin in Women With HER2/neu-Expressing Node-Positive or High-Risk, Node-Negative Operable Breast Cancer Open Protocol |
|
Protocol ID: BCIRG - 006
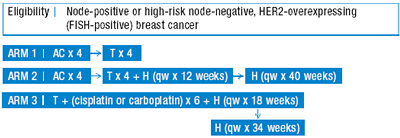
AC=doxorubicin/ cyclophosphamide, T=docetaxel
H = trastuzumab
Study Contact:
Linnea Chap, Chair, P h :310-206-6144
Jonsson Comprehensive Cancer Center, UCLA |
|
Select Publications
|
