| You
are here: Home: BCU 2|2003: Marc
L Citron, MD
Edited comments by Dr Citron
Evolution of eligibility criteria for CALGB 9741
trial
As a clinical oncologist and investigator, I wanted the eligibility
criteria to reflect the average breast cancer patient being treated
by an oncologist with adjuvant chemotherapy. I also wanted to make
it as easy as possible for oncologists to enroll patients.
The decision to accept a low baseline granulocyte count was very
important. For years in the treatment of testicular cancer, we would
not delay treatment because of a low count, when the goal of therapy
was cure. It made sense to apply this policy to adjuvant therapy
in node-positive breast cancer as well. In addition, we wanted to
increase participation by African-Americans, and benign neutropenia
occurs in approximately 10 percent of this population. We didn’t
want to exclude them. We also wanted the arms to be balanced to
prevent dose interruption and allow full-dose therapy. The easiest
way to achieve that was to allow a low absolute neutrophil count
(ANC) of 1,000.
Trial design and rationale for dose selection
We treated approximately 2,000 patients between four arms. The
drug sequence varied in each arm, but the drugs used and the dosages
were identical.
When this trial was designed in 1996, questions were raised about
the optimal doses and schedules for each of the drugs. NSABP B-22
had just shown no benefit to dose escalation of cyclophosphamide,
and CALGB 9344 was testing a higher dose of doxorubicin, so at that
point we thought it best to go with the standard dose. There was
also a lot of discussion about whether paclitaxel should be given
as a 24- or a three-hour infusion. But, it was important to design
the trial for outpatients. In addition, preliminary evidence from
the Gynecologic Oncology Group’s trial in second-line ovarian
cancer showed that those two types of infusion were equivalent.
The doses were pretty much derived from standard medical practice.

Efficacy of dose-dense chemotherapy
The trial had a two-by-two factorial design, and the results presented
at San Antonio compared the two dose-dense arms to the two sequential
arms. One disadvantage of the two-by-two analysis is that it precludes
pair-wise comparison of the two dose-dense arms. We will present
all four arms in the manuscript, but the dose-dense arms had similar
findings.
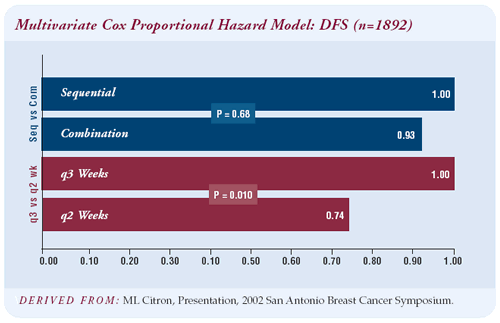
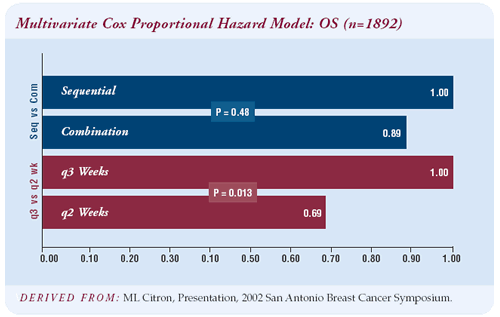
At a median follow-up of three years, dose-dense treatment was
associated with a 26 percent proportional reduction in relapse and
a 31 percent proportional reduction in mortality. We had expected
515 relapses based on CALGB 8541, the CAF dose-intensive trial,
however there were only 315 recurrences.
The four-year disease-free survival was 82 percent for dose-dense
therapy and 75 percent for the every-three-week regimens. I was
surprised by the magnitude of the difference — seven percent
at four years is significant. We’ll have to see whether the
survival benefit is lost or confirmed with further follow-up.
Most patients received the optimal doses of their drugs in all
arms, which may be related to the low ANC requirement and the fact
that less than eight percent of treatment cycles were delayed. This
assured us that the benefits of dosedensity could not be attributed
to a lower dose or further dose delays in the conventional regimens
— the arms were balanced in that regard.
Toxicity of dose-dense chemotherapy
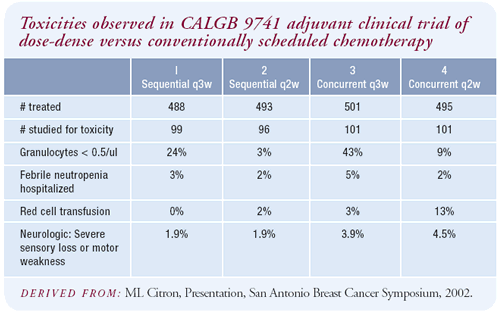
The advantages of dose density were not accompanied by an increase
in toxicity. In fact, the major difference in side effects was leukopenia,
defined as less than 500 granulocytes, which was significantly more
common in the every-three-week arms, with a P value of less than
0.0001. The incidence of hospitalization for febrile neutropenia
was also slightly higher in the everythree- week arms, but it was
uncommon in all arms.
Everyone was concerned about leukemia, but the results do not appear
different than the prior protocol, CALGB 9344, at the same exact
time point. The incidence is slightly less in the dose-dense arms,
although not statistically significant. Dose density also appeared
to have no impact on cardiac toxicity, which was less than two percent
in all arms.
For certain complications, we had information on only the first
100 patients in each arm. One of these was the incidence of red
blood cell transfusions, which was 13 percent on the concurrent,
dose-dense regimen, while only three percent or less in the other
arms.
This is difficult to understand, both from my experience in giving
dose-dense therapy and chemotherapy in general, because aggressive
use of red-cell stimulating factors generally prevents that complication.
This was the only major side effect seen with dose-dense therapy.
Interestingly, severe post-chemotherapy neurologic toxicity was
slightly greater in the patients who received concurrent chemotherapy,
whether it was every-two- or every-three-weeks. I can’t explain
that because we don’t consider cyclophosphamide to be neurotoxic.
It may be just a statistical quirk, but I’ve begun asking
my patients on AC if they’re having any neurological problems.
Occasionally I hear complaints of paraesthesias in those patients,
which I had previously attributed to dexamethasone. I’m watching
it more carefully now.
Effect of hormone receptor status on impact of
dose-dense chemotherapy
Because of the controversy regarding hormone responsiveness and
paclitaxel, there was concern that hormone receptor status would
affect responsiveness to therapy in this trial. However, there was
no significant difference based on receptor status with dose-dense
therapy. We did not plan this analysis, but because of the controversy,
we looked at that subset in retrospect. There was a 19 percent reduction
in patients with ER-positive disease and a 32 percent reduction
in patients with ER-negative disease. There was really no difference
— it works in both subsets.
A number of oncologists will not use ACT in ER-positive patients,
but I think dose-dense therapy can be applied to both ER-positive
and ER-negative patients with node-positive disease. I want further
follow-up from the study before I start using dose density in some
of the node-negative patients — generally I’m treating
them with an every-three-week regimen at this point. In lower-risk
patients with node-negative disease, I generally give AC times four.
Areas of future research in dose-dense therapy
We still need to verify the effect of dose-dense therapy, because
the two-by-two design doesn’t allow us to look at the individual
arms with sufficient power. We need another large trial that’s
not diluted by the two-by-two effect to determine the magnitude
of the difference between dose-dense and conventional dosing. We
also need to refine the four arms to prove which has the highest
cure rate. That would be my next step.
There are a number of other ways to study dose density. The fact
that sequential versus combination therapy appears to be equivalent
opens up the feasibility of studying a number of therapies sequentially
— chemotherapy, monotherapy and biological therapies —
in a potentially curative manner. We didn’t do a quality-of-life
companion in this trial, but based on my experience, it stands to
reason that one drug is less toxic than two, and that sequential
therapy will probably be better tolerated in the older age group.
This needs to be studied because the ability to give full-dose chemotherapy
in the elderly is important.
Another issue to consider is further decreasing the dose-dense
interval, so that you treat again as soon as the monocytes recover.
It’s a little more difficult to consider this in the AC arms,
because AC may cause esophagitis and other problems that may be
more difficult to manage if associated with neutropenia.
Acceptance of dose density in clinical practice
Dose-dense therapy is definitely a therapeutic option for high-risk
patients with breast cancer at this time. It is not the standard
of care, but an alternative to discuss with patients at risk for
relapse within the next three or four years. In my older patients,
who may not be able to tolerate combination treatment, I use sequential
ATC, and I think we’ll find sequential, dose-dense ATC will
be tolerated well by the elderly.
I always present patients with their options, and I like to hear
what they have to say. In general, patients want the treatment with
the most potential for cure. Many also want to receive the treatment
quickly — in fact, that’s one of the most common reasons
patients express for wanting dose-dense therapy. I was initially
embargoed from revealing the results of CALGB 9741, but now I discuss
it with patients. I give them my take on the literature and my recommendation.
Most oncologists like to see five-years of follow-up in an adjuvant
study. I find when I talk to physicians about emerging trends, you
can generally divide the reactions into thirds. One third embrace
it, a second third are not sure and the remaining third are definitely
against it. I’ve been surprised how positively dose-dense
therapy has been received. As I talk to physicians, I find they
are often already using or at least considering it. This approach
appears to be more widely accepted than I had expected at this time.
Personal reflections on oncology research and
practice
I love oncology — I have a great practice, terrific patients,
and a great staff. I enjoy my work. I am tired at the end of the
day, like everyone else, but I almost always feel good about it.
And I love research. Preparing the paper for CALGB 9741 has been
a very interesting experience for me, and I’ve been totally
immersed in it for about six months. I enjoy chess and, like chess,
research is an enjoyable, intellectual challenge. For years, I worked
in a laboratory studying DNA repair, and I always found it interesting
to study basic mechanisms and then design clinical experiments.
Select publications
|
