| You
are here: Home: BCU 2|2003: Charles
L Vogel, MD, FACP
Edited comments by Dr Vogel
Rationale for the design of the trastuzumab monotherapy
trial in metastatic disease
It became readily apparent to me early on that there was a subset
of women with metastatic HER2-positive disease who really did not
want to receive chemotherapy up front, so I lobbied for having a
first-line, single-agent trastuzumab trial. Many other investigators
— including Melody Cobleigh and Debu Tripathy — were
also very instrumental in moving this concept forward. So, this
was really the third major initial trial to look at what trastuzumab
could do in metastatic breast cancer. All of these were basically
proof-of-principle trials. While everybody was entering into these
trials with some degree of optimism, it wasn’t a "slam
dunk" that trastuzumab was really going to provide an important
benefit.
Our trial was a Phase II, single-arm study, and we accrued 114
patients. The patients were quite gratified because they were treated
with a relatively nontoxic form of therapy, at least from the standpoint
of subjective toxicities.
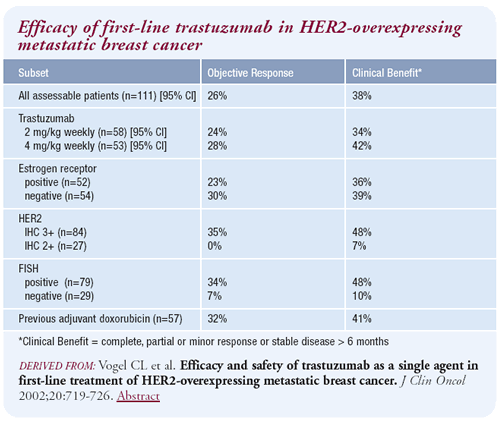
Results of the trastuzumab monotherapy trial
The overall, published response rate for all the IHC 2+/3+ HER2-positive
patients was 26 percent. We’ve subsequently learned that there
is a very high false-positive rate for the IHC 2+ patients. Consequently,
further analyses were done using only the IHC 3+ patients, and ultimately,
the FISH-positive patients.
Another interesting outcome measurement is prolonged stable disease,
because it seemed that patients were responding to trastuzumab more
like they would to hormonal therapy rather than to chemotherapy.
We were seeing prolonged periods of disease stabilization, even
though we weren’t able to objectively record definitive responses,
as classically defined. So, we also evaluated the group of patients
with prolonged stable disease for greater than six months.
If you look at the group of patients who were FISH-positive, and
if you add the patients with prolonged stable disease to those who
had objective responses, about half the patients responded to first-line,
single-agent trastuzumab.
According to strict statistical guidelines, we observed a median
duration of response of 18 months. That is far in excess of what
was seen in the pivotal trial, and it could very well be that a
patient here or there could have skewed that result.
Management of patients with HER2-positive metastatic
disease
There remains considerable controversy regarding the optimal method
to routinely evaluate HER2 status. I won’t treat a patient
with metastatic breast cancer until I have a FISH assay. In the
June 2002 issue of the Journal of the National Cancer Institute,
the NSABP and the Intergroup published their experiences with HER2
assessment, and it really cast doubt about our quality control for
immunohistochemistry. Until the American College of Pathology does
something to iron out this problem of quality control, I continue
to use FISH.
There is a significant survival benefit for the combination of
trastuzumab plus chemotherapy versus chemotherapy alone. For that
reason and because some of the patients might wish to avoid cytotoxic
chemotherapy at that point in time, it behooves us to move in the
direction of ascertaining HER2 status prior to initiation of first-line
chemotherapy.
I use single-agent trastuzumab in a similar manner as hormonal
therapy. There are subsets of women with HER2-positive disease who
don’t have horribly aggressive metastatic breast cancer. In
those relatively asymptomatic patients who do not have visceral
crisis or rapidly progressive disease and are not incapacitated
by symptoms, I have no problem at all starting them on first-line,
single-agent trastuzumab. However, the patients must be fully informed
that they may be giving away something in terms of response rate,
based on an analysis of crosstrial comparisons with the combination
regimens.
Management of the patient with ER-positive, HER2-positive
metastatic breast cancer
Although it's controversial, some physicians use the combination
of trastuzumab and hormonal therapy off-protocol for HER2-positive,
hormone receptor-positive patients. I don’t use that combination.
Hormonal therapy is the mainstay of treatment and can produce prolonged
responses. It’s very important to know whether a patient has
hormone-sensitive disease. I would not like to cloud the issue by
adding trastuzumab until such time as the ongoing clinical trials
are published and indicate a definite advantage for the combination
versus the sequential approach.
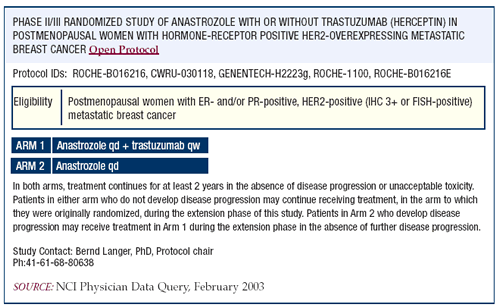
There's a worldwide trial that has been accruing very, very slowly
that compares anastrozole with or without trastuzumab. Everybody
underestimated the difficulty that would ensue with this particular
type of protocol. Approximately 20 percent of tumors will be FISH-positive,
and of those, perhaps 40 percent will be ER-positive. Now you’re
already down to less than 10 percent of the overall breast cancer
population. The eligibility criteria carve away another few percent.
And so you’re down to probably about seven percent of the
overall patient population who could potentially be eligible for
such trials. It’s not at all surprising that there is difficulty
accruing to these types of trials.
A palliative approach to women with HER2-positive
metastatic disease
Until we are able to break the "cure barrier" of metastatic
breast cancer, the major philosophical goal is palliation. I don’t
necessarily know that anthracyclines, or even taxanes, are the best
chemotherapeutic agents for palliation of metastatic breast cancer.
In this day and age, patients are frequently treated with an anthracycline
plus docetaxel as first-line treatment in metastatic breast cancer.
That's counter to my own personal philosophy of the management of
metastatic breast cancer. An anthracycline/docetaxel combination
is a very reasonable combination for a patient with visceral crisis.
However, the vast majority of patients don’t present with
visceral crisis. Consequently, if I had a patient who was HER2-
positive, I would want to "put my best foot forward" from
the standpoint of toxicity.
I might consider, as a first choice, vinorelbine plus trastuzumab.
The weekly taxanes combined with trastuzumab are also reasonably
good options. And here, I would probably choose paclitaxel over
docetaxel, because even with weekly docetaxel, we run into problems
with the epiphora. And we still have the fluid-retention problem
with docetaxel, more so now with the weekly than even the every-three-week
schedule.
On the other hand, we now have new data, both from the pilot trials
of the BCIRG and also from the study just presented at San Antonio
by Nick Robert for the US Oncology group, on the use of carboplatin
plus paclitaxel and trastuzumab. The duration of response in those
trials appears to be, in crosstrial comparisons, superior to those
seen with trastuzumab in combination with vinorelbine, paclitaxel
or docetaxel.
We use that combination in our practice and are actually studying
that in the neoadjuvant setting. If I had a patient with visceral
crisis who was HER2- positive, I would strongly consider using a
combination of carboplatin, probably with docetaxel and trastuzumab.
Treating the patient with ER-negative, HER2-negative
metastatic breast cancer
The patient with hormone receptor-negative, HER2-negative de novo
metastatic disease constitutes a major dilemma. Sometimes these
patients have really minimal disease.
Whenever possible, I like to try to observe these patients, as
opposed to starting cytotoxic chemotherapy, because I’m really
not convinced that the early institution of cytotoxic chemotherapy
is going to lead to a survival advantage. It is likely to impact
negatively on quality of life. On the other hand, the vast majority
of women, once they know they have metastatic disease, are not going
to accept the concept of observation.
My next step would be to try to find a nontoxic clinical trial
that I could enter the patient on — the new targeted therapies
or the biologics. When it comes to cytotoxic chemotherapy, my choice
would not necessarily be an anthracycline and probably not even
a taxane.
I’m impressed with the tolerability of and response to single-agent
therapy with capecitabine, vinorelbine, liposomal doxorubicin or
gemcitabine. CMF is also a well-tolerated regimen. Another regimen
that we use is a combination of mitoxantrone, 5-FU and leucovorin,
where the 5-FU and leucovorin are given on days one and eight and
then mitoxantrone on day one. This regimen is not utilized by very
many people, but you can obtain very nice responses with minimal
toxicity.
Several questions arise. How do you choose among all of these
different options? Do you choose a combination? Do you choose sequential
single agents? Unless the patient is part of a clinical trial where
we’re trying to find such significant activity for a combination
that it could be moved into the adjuvant setting, I would probably
use sequential single agents.
Vinorelbine/capecitabine (VINOCAP) for patients
with ER-negative, HER2-negative metastatic disease
For patients where you might need a little bit more "bang
for your buck," I consider a combination of vinorelbine and
capecitabine, where we’ve seen excellent responses. There
are at least six Phase II trials — all of them concordant
— showing excellent response rates with good tolerability
for that particular combination.
Our group now has about 24 patients in an observational study
evaluating the combination of vinorelbine and capecitabine. There
were some patients receiving up to fourth-line chemotherapy. We
found that the overall response rate was about 56 percent, which
was quite credible. Toxicity was minimal at what might be considered
virtually homeopathic doses of both of the drugs.
The median dose intensity for vinorelbine was about 18 mg/m2 per
week, whereas the standard dose is either 25 or 30 mg/m2 per week.
In reality, whatever study you look at — because of the myelotoxicity
— the median dose intensity is usually about 21 to 22 mg/m2
per week.
In our particular series, the dose intensity was actually even
lower. With regard to the dose intensity of capecitabine, all of
our patients were treated with a median dose intensity of about
1,500 mg/m2 per day. So, we’re talking about relatively low
doses of these two agents.
VINOCAP: Toxicity profile
There’s very little alopecia associated with the vinorelbine/capecitabine
combination. One disadvantage is that we don’t give —
in our particular practice setting — vinorelbine without a
portacath. Many people do, both in the United States and abroad,
but every once in a while you see a patient with a significant arm-pain
syndrome. And when you’ve had one or two patients with this,
you really do not want to repeat it. You very seldom see a paralytic
ileus with vinorelbine, but certainly, when this occurs, you start
to become "gun shy," because it’s a very serious
complication.
The major side effect of this combination is neutropenia from
the vinorelbine, and, as long as the doses are low enough from the
capecitabine, you really should see very little diarrhea, mucositis
or hand-foot syndrome.
Nonprotocol adjuvant decision-making in the patient
with ER-negative, HER2-negative, node-positive breast cancer
The presentation in San Antonio of the dose-dense chemotherapy
study by Mark Citron on behalf of the Intergroup was fascinating
and provides vindication for the mathematic modeling that Dr Norton
and his group have been espousing over the years. I know that many
of the cooperative groups around the world will want to use this
to generate hypotheses. We have other regimens that also look quite
good. The combination of docetaxel, doxorubicin and cyclophosphamide,
or TAC, appears to provide results that are very similar to the
dose-dense results of the Intergroup. So, what do you do? Do you
just abandon the TAC regimen and adopt dose-dense therapy on the
basis of one clinical trial? I wouldn’t be prepared to do
that just yet.
And then the question is: Could you do even better with dose density
if you used a different taxane? Unfortunately, we still don’t
have good head-to-head comparisons among the taxanes, and we’re
still waiting for the major pivotal trial of the Intergroup, which
is AC followed by either docetaxel or paclitaxel, with both of those
drugs given either every three weeks or weekly. That is a major
study that will impact yet further on taxane usage. In addition,
there are 56,000 women worldwide who are entered into adjuvant taxane
trials. Over the next two to three years, those trials will mature
and we’ll know a lot better what to do with the taxanes.
Outside of the context of a clinical trial, I would give patients
three options. I would tell them they could take AC followed by
docetaxel, TAC or now — with the new data presented at San
Antonio — I might present the possibility of using a sequential
single-agent dose-dense chemotherapy regimen. However, I would make
a leap of faith and substitute docetaxel for paclitaxel. I can’t
assure patients that sequential single-agent, dose-dense therapy
is going to be the best option, but on the basis of cross-trial
comparisons, it looks like it’s not too dissimilar from the
results one can obtain with TAC.
Adjuvant therapy for patients with ER-positive
breast cancer
After the ATAC trial results were initially presented, I began
speaking with my patients about the results. I told them that the
data were still early, and we discussed the risk-benefit profile
and quality-of-life issues. Then they made the decision to receive
anastrozole or tamoxifen.
There are some women who are so concerned about the potential for
osteoporosis with anastrozole, or those who already are suffering
from arthritic symptoms, that they will say, “If those side
effects are predominant with anastrozole, then I’d prefer
to go with tamoxifen.” On the other hand, women who are deathly
afraid of the uterine cancer risk and blood clots may choose to
go with tamoxifen. At the moment, both of these are still very viable
options.
Integrating fulvestrant into the management of
patients with ER-positive metastatic disease
We treated 21 patients in the compassionate-use trial and subsequently
another 14 patients after fulvestrant became commercially available.
Tolerability is no problem and is comparable to the aromatase inhibitors.
Patients also deal quite well with the intramuscular injection.
I really don’t know where fulvestrant will "shake out"
over time. We know that fulvestrant is at least as efficacious as
the aromatase inhibitors. It will be very nice if fulvestrant does
have the very prolonged duration of disease control, as initially
published.
Select publications
|
