| You
are here: Home: BCU 2|2003: Peter
Ravdin, MD, PhD
Edited comments by Dr Ravdin
ATAC trial data update
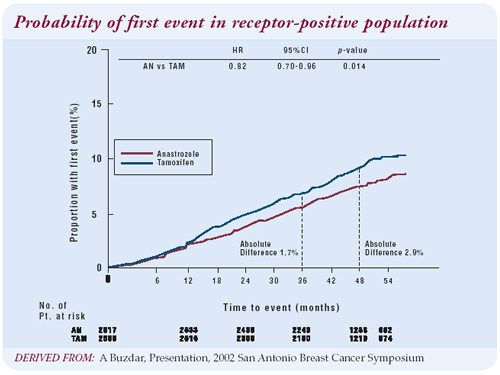
After 47 months, the disease-free survival data from the ATAC
trial look very good. The curves continue to diverge, and there
is no sign that they are coming together. It’s like a primary
election with 80 percent of the precincts reporting, and a pretty
solid lead for a candidate. It’s very likely that in five
years, the anastrozole arm will be superior in terms of relapse-free
survival. Given its current lead, it’s virtually impossible
that it won’t be.
Tamoxifen only reduces mortality by about 30 percent, so there’s
a lot of room for improvement. At this time, no mortality data on
anastrozole has been presented. It is possible that because of the
kind of relapses anastrozole prevents that there won’t be
as great an effect on survival as on disease-free survival. However,
if it has a large effect on either, it would still be considered
beneficial.
ATAC trial data update: Impact on clinical care
Until now, I had not changed my clinical practice based on the
early ATAC results. I was waiting to see more data and whether or
not the curves were coming together. However, at 47 months, the
divergence of the curves shows a three-percent advantage for anastrozole.
There will not be three-percent events in either arm over the next
year; therefore, the anastrozole advantage will continue to be the
same or greater in the next year.
I will now tell patients that there are two options. One option,
tamoxifen, seems less efficacious in the short-term, but we know
its short- and long-term toxicities. With anastrozole, the time
to relapse is substantially improved at the four-year point, but
we really don’t have any long-term safety or efficacy data.
The FDA did, however, find adequate evidence to allow approval of
the drug in the adjuvant setting. There is a risk with either therapy,
and some patients will want the new therapy with the potential to
be better.
I’m particularly tempted to use anastrozole in patients at
increased risk for a thrombotic event. I include age as a risk factor,
because, for example, a 65-yearold woman has a one- to two-percent
chance of a major thrombotic event over the next five years. Anastrozole
would not elevate that event rate; therefore, if the patient has
good bone mass, placing her on anastrozole becomes a safety issue,
regardless of whether there is improved efficacy.
Anastrozole and bone loss
The updated safety data still shows that anastrozole is better
in terms of thrombotic events and endometrial cancer, but worse
in terms of fractures. I would like to see more mature safety data.
There is an important carryover toxicity effect that might be
expected after the drugs are stopped. For example, tamoxifen has
a carryover effect in endometrial cancer risk for five years after
therapy. The real question with anastrozole is whether the bone
loss patients experience during therapy increases their risk for
the rest of their lives.
I’m not half as worried about osteoporosis as I am about
thrombotic events with tamoxifen. Osteoporosis is like watching
a hurricane come in from Africa — you can prepare for it,
predict where it’s going to hit and do something to make yourself
less vulnerable to its effects. In patients I start on adjuvant
anastrozole, I routinely check baseline bone mineral density to
determine which patients need to be monitored soon or started on
a bisphosphonate to protect bone mass.
Prevention of bone loss: Bisphosphonates
Bone loss can be managed. Dr Gnant presented a study at San Antonio
looking at ovarian ablation with anastrozole versus tamoxifen and
bisphosphonates. They saw protection from bone loss by adding zoledronate.
In addition, the women who received tamoxifen and ovarian suppression
without a bisphosphonate had a drop in bone loss, which was corrected
when they got zoledronate.
The data presented by Dr Gnant is important with regard to anastrozole
because without agents like zoledronate, osteoporosis would be a
major issue. But this study showed that bisphosphonates have the
potential to totally prevent the risk of bone loss.
Advantages to a well-powered trial
It is heartening that the number of women in the ATAC trial was
greater than the number of postmenopausal, ER-positive women who
received five years of tamoxifen in the overview. I applaud the
ATAC trial because it is enormously overpowered to determine which
drug is better. The beauty of a trial being overpowered is that
you can begin to ask subset questions — that’s the power
of the overview.
I also like overpowered trials because they are almost impossible
to replicate. For example, ATAC may be the only chance to ask what
predicts for benefit with tamoxifen or anastrozole, because if survival
and toxicity advantages emerge for the anastrozole arm, it may be
impossible to do such a trial again and ask the biological questions
as corollaries to it. To do it all in one 9,000- patient study is
immensely powerful. This trial will never be replicated.
Use of other aromatase inhibitors in the adjuvant
setting
I do not use letrozole for adjuvant therapy in the nonprotocol
setting. It’s probably equivalent to anastrozole, but I don’t
see any significant advantages. If there was a problem with anastrozole,
it would have shown up in this study of 9,000 patients, and I would
be able to warn my patients or switch them if necessary. With letrozole,
I have no way of knowing if there’s an issue.
I have been looking at whether exemestane might have some advantages
compared to anastrozole. There will be trials to test this. Exemestane
is a very different aromatase inhibitor — it’s irreversible
and it has a steroidal structure. Early laboratory evidence suggests
it will not be associated with bone loss.
The resistance mechanisms of exemestane might also be different,
which could be both better and worse. Remember that tamoxifen can
actually be read as an estrogen. I’m curious to see if a drug
with a steroid backbone, such as exemestane, might also be interpreted
in some systems as an estrogen. Perhaps the same resistance mechanisms
that cause resistance to tamoxifen might also cause resistance to
exemestane.
Calculating expected benefit from therapy
The informal way to calculate a patient’s expected benefit
is to multiply her risk of recurrence by the relative risk reduction
expected from a therapy. For example, if a patient has a 60 percent
risk of recurrence and your therapy reduces that by 40 percent,
multiplying those two percentages together gives a 24 percent benefit.
This is a rough approximation, and it doesn’t quite work
that way. In reality, the 40 percent reduction is not a 40 percent
composite reduction, but rather a 40 percent reduction that occurs
in each year. It compounds like interest in a bank account and actually
ends up giving less of an effect than you would expect.
The reason is mathematical, not biological. On a curved surface,
you can’t lay your ruler down across the entire curve. Rather,
the ruler is essentially touching the curve at any given point and
telling you what the slope is at that point. That’s what the
hazard is — it tells you what the actual ratios of the slopes
would be if you put the ruler at four years on both curves.
I wrote a program on adjuvant therapy that shows how the numerical
method for calculating benefit works. It’s accessible online
at www.adjuvantonline.com.
Small reductions may offer big benefits
A two percent absolute difference sounds modest, but it can be
important. The overview suggests that the proportional benefits
hold up for a given therapy, irrespective of the baseline risk.
If low-risk patients benefit 20 percent from a given therapy, high-risk
patients receive a 20 percent relative benefit as well. So for the
low-risk patient, a 20 percent benefit may be only one or two percent.
But for the high-risk patient with a 50 percent risk, a 20 percent
difference is a 10 percent risk reduction. This therapeutic index
gets higher and higher with risk.
I would not expect any therapy to be effective in 100 percent
of patients because breast cancer is a very heterogeneous disease.
Patients can have ER-positive and -negative disease or HER2-positive
and -negative disease. Different cancers express different genes
and, therefore, have potentially different vulnerabilities to therapy.
I would be amazed if any single therapy was effective in more than
50 percent of patients. Given that, when you see a 20 percent effect,
an additional two percent represents perhaps 20 percent of that
overall population, which is significant. And perhaps that particular
agent benefits 20 percent of the patients who aren’t benefiting
from other strategies.
I believe the conquest of breast cancer is not going to be one
magic bullet, but rather identifying sets of patients whose cancers
have specific vulnerabilities. Maybe none of those sets will be
greater than 20 percent of the total, so even the greatest therapies
may be very effective only for a small set of patients.
Impact of therapy on early versus late relapses
The divergence of curves with effective adjuvant therapy has not
been adequately studied, and I think there is an enormous hidden
story there. Some curves begin to diverge within the first year,
continue to diverge for the first five years and then parallel each
other. Curves like this tell me the therapy is killing the rapidly
progressive, early relapsing clones.
The last overview showed that the proportional benefits for chemotherapy
emerged entirely because of impact on relapses within the first
five years. There was no impact at all on relapse from the average
chemotherapy after five years — a fascinating result.
With chemotherapy, we are not yet touching the late, slowly proliferating
population, which accounts for perhaps one-third of all relapses,
particularly in ER-positive disease. This is where vaccines may
be of particular benefit. In contrast, there was a curve for a particular
therapy presented at San Antonio that showed no difference in the
first five years, but the advantage accumulated in the second five
years. The curve suggested the therapy showed no advantage against
the rapidly progressive clones in the early relapsers, but that
the advantage emerged in the late relapsers.
Hormone therapy is more balanced than chemotherapy in the impact
on the second five years. In NSABP P-1 and B-14, the curves actually
slightly diverge. The therapy is probably acting on the slower and
stalled clones. This has not been adequately studied, and I think
it’s worth some additional research.
CALGB 9741 results and the impact on clinical
care
I think the results of CALBG 9741 will pressure physicians to
introduce the dose-dense regimen. Many clinicians will want to obtain
some experience with dose density in their very high-risk patients,
who might have received very high-dose chemotherapy with stem-cell
support in the past. For those patients, these new dose-dense regimens
are extremely interesting.
The problem is that clinicians will want more information about
the toxicity profile. There was evidence that the sequential, noncombination
dose-dense regimen was less toxic. Many of us are leery about giving
very large cumulative doses of doxorubicin, so this will take some
soul searching. I don’t think physicians are going to suddenly
adopt one of the dose-dense regimens tomorrow morning; rather, I
think they will ease into it.
Palliation versus cure for metastatic disease
Physicians generally agree that the targets of treatment for patients
with metastatic disease are long-term health maintenance and disease
palliation. Today, we don’t talk about intensive therapy for
cure. At San Antonio five years ago, there would have been discussions
about high-dose chemotherapy with stem-cell support. Now we discuss
the breadth of single agents available to support patients. We’re
looking to maintain patients’ function for prolonged periods,
rather than to induce cure.
Physician acceptance of capecitabine
Kathy Miller presented a case at a seminar recently in which the
patient progressed shortly after receiving adjuvant ACT. When the
members of the audience were asked what agent they would use next,
the most common answer was capecitabine alone. I was a surprised
by that because the acceptance of capecitabine was slow in the beginning.
I attribute this slow acceptance to two factors. First, capecitabine
was approved at too high a dose; so many physicians had an unfavorable
first experience using it. Second, capecitabine is the first drug
I can think of that was approved before there were any publications
in the literature.
Physician acceptance has grown as lower doses have been tried
and patients’ tolerance has improved. In addition, articles
have suggested a relatively high response rate with capecitabine
as first-line therapy and in combination therapy, particularly with
docetaxel.
Capecitabine/docetaxel combination in the adjuvant
and metastatic settings
The capecitabine/docetaxel study was important because a very
large number of patients consider combination adjuvant therapy.
Therefore, the most valuable outcome of this trial in the metastatic
setting was learning whether or not this combination might have
promise in adjuvant therapy. Patients with metastatic disease in
really desperate situations might have a high response rate with
the combination, but that’s not the majority of patients.
Most patients don’t relapse, and those who do don’t
usually find themselves in a desperate situation early on.
If there had been a sequential arm of capecitabine followed by
docetaxel, I don’t think we would have seen a great deal of
difference between the two arms, as in many other crossover studies.
Select publications
|
