| You
are here: Home: BCU 6|2003: Joyce
O’Shaughnessy, MD
Edited comments by Dr O’Shaughnessy
Adjuvant/neoadjuvant trials incorporating capecitabine/
docetaxel (XT)
The ongoing US Oncology adjuvant clinical trial is for patients
with nodepositive or high-risk, node-negative breast cancer. Patients
are randomized to AC x 4 cycles, followed by four cycles of docetaxel
with or without capecitabine x 4 cycles. There are two other trials
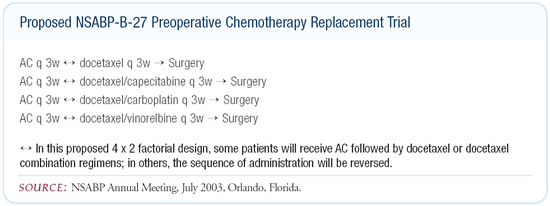
evaluating the XT combination: the proposed replacement study for
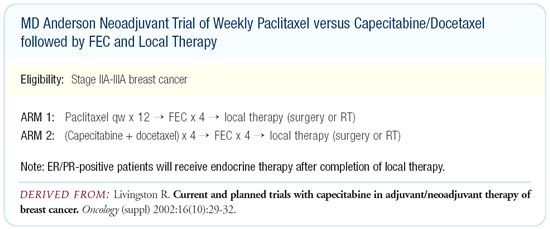
NSABP-B-27 and the MD Anderson ongoing neoadjuvant trial in which
patients are randomized to weekly paclitaxel for three months versus
the XT combination with both arms followed by FEC.
In a nonprotocol setting, my standard adjuvant approach is AC
followed by docetaxel. I believe duration is important, especially
for patients at high risk — patients with large tumors or
positive nodes, particularly those with macrometastases in the
nodes. When treating women at very high risk, I stick with the
data as much as possible and use the CALGB FAC regimen at 600/60/600
mg/m2 followed by docetaxel at 100 mg/m2.
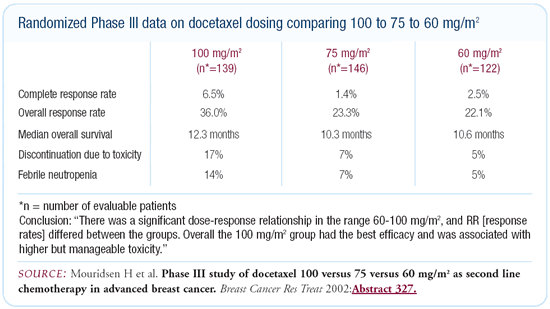
The data from San Antonio comparing three different dose levels
of docetaxel — 100 mg/m2 versus 75 mg/m2 versus 60 mg/m2 — resulted
in response rates of 36 percent versus 23 percent versus 22 percent,
respectively. That’s a 50 percent improvement in response
rate, which may translate into improved disease-free and overall
survival. If I had breast cancer and was treated with docetaxel,
I would want 100 mg/m2.
Utilization of microarray profiles to predict
pathologic response
In their neoadjuvant study, MD Anderson will utilize microarray
technology to perform gene transcription profiling on fine needle
aspirates. They will then correlate the differential gene expression
with pathologic responses. Dr Pusztai has compared core biopsies
to fine needle aspirations (FNAs) and found the microarrays perform
equally well. If we can define a microarray signature that predicts
a pathologic complete response — and apparently there’s
been some recent success in doing that — that would be very
exciting.
We will work very closely with Dr Pusztai to repeat that trial
with a different set of chemotherapy drugs which we anticipate
to be FEC followed by capecitabine/docetaxel as neoadjuvant therapy.
We want to see if we can correlate the microarray profiles on the
primary tumor — also collected through fine needle aspiration — to
determine the sensitivity and specificity for a microarray pattern
predicting whether a patient will have a pathologic complete response
or not.
Evaluating molecular markers in clinical trials
of capecitabine
There are ongoing clinical trials evaluating protein and mRNA
of thymidine phosphorylase (TP), dihydropyrimidine dehydrogenase
([DPD], which is the key catabolic enzyme for 5-FU), and thymidylate
synthase ([TS], which is the target enzyme for 5-FU).
Preclinical and xenograph data demonstrate that the likelihood
of responding to capecitabine is related to the ratio of TP to
DPD. In animal models, the higher the TP level and the lower the
DPD level, the more likely there will be a response to capecitabine.
I think capecitabine may be one of the first chemotherapy agents
we’re able to select based on protein and mRNAs.
Impact of CALGB-9741 dose-dense data on clinical
practice
CALGB-9741 was a well-conducted trial, and with the hazard ratio
for recurrence of 0.5 at four years, I believe the data will hold
up. Dose-dense scheduling resulted in an 82 percent, four-year,
disease-free survival rate.
That is exactly the same as the three-year, disease-free survival
rate achieved with TAC when compared to FAC. Dose density has become
an option for patients and completing therapy more quickly may
be beneficial. However, there are no data to prove that a dose-dense
regimen is superior to TAC or to AC followed by docetaxel.
In the adjuvant setting, if you are going to administer AC followed
by paclitaxel, you should probably use the dose-dense regimen.
However, we need to know whether it’s true that by cycling
agents every two weeks, we produce more cell kill, as the Norton-Simon
hypothesis suggests.
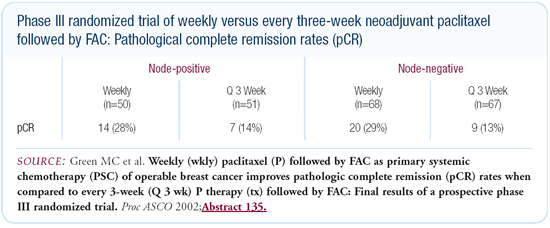
Marjorie Green’s data from MD Anderson, comparing weekly
paclitaxel preoperatively to every-three-week paclitaxel — both
followed by FAC — showed a doubling of the pathologic CR
rate with weekly paclitaxel. Many oncologists believe that part
or perhaps all of the benefit seen in CALGB-9741 could be explained
by administering paclitaxel alone every two weeks, so it’s
not at all clear that AC should be given every two weeks.
In my practice, I have not yet incorporated dose-dense chemotherapy
because I believe duration is important. I want to cure women,
and I’m going to administer an agent for whatever duration
I believe is necessary.
Impact of CALGB-9741 dose-dense data on clinical
trial design
We would like to improve upon the CALGB-9741 data, and our clinical
trial of AC followed by docetaxel versus capecitabine/docetaxel
may result in the next big advance in adjuvant therapy. The US
Oncology Breast Committee discussed giving docetaxel every two
weeks, but there’s no real rationale for doing that. It has
a long half-life and capecitabine is already dose-dense because
it’s administered for two weeks followed by one week off.
We didn’t feel the need to manipulate that aspect of the
clinical trial.
We considered administering AC every two weeks, but we don’t
have any data that it is more beneficial than an every-three-week
schedule. There was a neoadjuvant trial presented in San Antonio
by Euler and colleagues evaluating three cycles of epirubicin/cyclophosphamide
120 mg/m2 and 600 mg/m2, respectively, randomizing between administering
the combination every three weeks versus every two weeks. The pathologic
CR rate in the every-three-week group was 9.5 percent compared
to 3.9 percent in the every-two-week group. With only 262 patients,
it’s not a definitive study, but it certainly doesn’t
suggest that giving EC every two weeks is better.
Our US Oncology Breast Committee concluded that we would watch
accrual to our trial, and doctors will “vote with their feet.” Currently
we’re accruing between 65 and 75 patients a month, and we
haven’t seen a big drop-off since the San Antonio meeting.
Dose-dense scheduling of docetaxel
Approximately one-third of practitioners are using dose density
in their practice. Interestingly, a question was posed at a meeting
with community oncologists: “ Would you use dose-dense AC
followed by a taxane every two weeks?” Onethird responded
affirmatively. When asked: “What taxane would you use?” approximately
55 percent indicated docetaxel and 45 percent chose paclitaxel.
It is premature to use docetaxel every two weeks without more
safety and efficacy data. You can administer docetaxel 100 mg/m2
by itself and it’s relatively safe. It’s really not
feasible to administer docetaxel 100 mg/m2 every two weeks following
AC because there’s too much skin toxicity. I don’t
know what you would use for dose-dense therapy in the neoadjuvant
or adjuvant setting because we just don’t have enough data.
Phase III trial of nanoparticle paclitaxel versus
paclitaxel
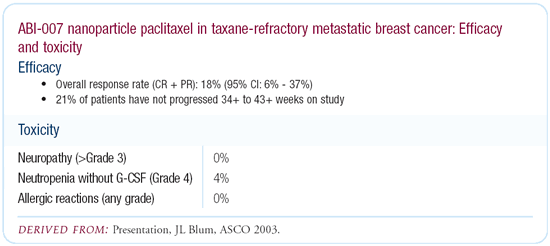
I’m closely watching the randomized Phase III trial of
paclitaxel 175 mg/m2 every three weeks versus ABI-007 260 mg/m2
every three weeks. ABI-007, or nanopaclitaxel, is an albumin-formulated
paclitaxel. It’s cremophor-free, so you can raise the dose
safely, and it’s very well-tolerated.
In our Phase II trial of weekly ABI-007 100 mg/m2, three weeks
on, one week off, in taxane-refractory patients, we’re seeing
some very impressive responses and extremely good tolerability.
If that’s a positive trial, as it may well be, it will be
a very important advance because you won’t need steroids.
In my experience, the neurotoxicity with 100 mg/m2 is negligible.
CALGB trial: Adjuvant capecitabine versus CA
or CMF in elderly women
We did a small, randomized Phase II trial comparing intravenous
CMF and fulldose capecitabine as front-line therapy in elderly
patients in the metastatic setting. The response rate with capecitabine
was 30 percent compared to 16 percent with intravenous CMF.
In a randomized Phase II trial of patients pretreated with anthracycline,
comparing paclitaxel 175 mg/m2 every three weeks to full-dose capecitabine,
1,250 mg/m2 BID, two weeks on, one week off, the response with
the capecitabine was 36 percent compared to 26 percent with paclitaxel.
The confidence intervals were widely overlapping, so we couldn’t
conclude that capecitabine is superior, but what you can say from
these two studies is that it’s certainly unlikely that capecitabine
is worse than CMF or paclitaxel.
It’s interesting how quickly capecitabine has moved to
trials in the adjuvant setting. In women over age 65, 75 percent
have ER/PR-positive breast cancers. I think the role of chemotherapy
in that group of patients is sufficiently unknown. For women over
70, in particular, there are so few patients in the overview analysis
in that age group that I think it’s very reasonable to compare
capecitabine to AC or CMF. I’d be a little less comfortable
with it in a younger patient population, only because the overview
has clearly shown that polychemotherapy is superior to monotherapy.
Fulvestrant in the treatment of postmenopausal
ER/PR-positive metastatic disease
I’ve used a fair amount of fulvestrant, and it’s
very well-tolerated. We’ve had some very nice responses to
fulvestrant, including one of my patients who was on the original
clinical trial of fulvestrant versus anastrozole. She was on fulvestrant
for three and a half years and now she’s on anastrozole.
The injections have not been an issue for patients, and most women
are very grateful that the side-effect profile is close to nil.
I think fulvestrant probably crosses the blood-brain barrier and
patients do have hot flashes on it, but in general, they’re
quite mild.
I am a little disquieted by the fact that it can take three to
five months to reach a steady state with fulvestrant. A patient
with rapidly progressing disease may not benefit from fulvestrant,
but fortunately most women with hormoneresponsive breast cancer
have relatively indolent disease. I’m very interested in
the clinical trial in which they are loading fulvestrant 500 mg
every two weeks for a couple of doses and then reducing it to 250
mg monthly. That makes sense to me, so I’ve been trying to
load it a little by giving it every three weeks for several injections
in an attempt to raise the levels more quickly.
Sequencing hormonal therapies in the front-line
treatment of metastatic breast cancer
When I see a postmenopausal patient who has relapsed on adjuvant
tamoxifen, I tend to use an aromatase inhibitor followed by fulvestrant
when the disease progresses. In the frontline metastatic trials
of aromatase inhibitors versus tamoxifen, data demonstrates that
regardless of whether you administer an aromatase inhibitor after
tamoxifen or tamoxifen after an aromatase inhibitor, there’s
a 40 to 50 percent clinical benefit rate. In the second-line setting,
if you administer fulvestrant after an aromatase inhibitor or an
aromatase inhibitor after fulvestrant, there’s approximately
a 33 percent clinical benefit rate. These are all small trials
and most of them are not randomized, but they show that any of
these regimens can be effective and there’s no mandatory
sequence for these agents.
Sequential single-agent versus combination chemotherapy
for metastatic disease
George Sledge’s Phase III trial of single-agent doxorubicin,
paclitaxel versus the combination of doxorubicin/paclitaxel as
front-line chemotherapy for metastatic breast cancer failed to
show a survival benefit for the combination. It’s difficult
to demonstrate a survival advantage in front-line metastatic disease
because, according to the MD Anderson series, these patients live
an average of four years. What you do early in their treatment
may never be reflected in a survival advantage because they have
many other opportunities for treatment down the line.
In chemotherapy-naïve patients with metastatic disease,
I generally use capecitabine/docetaxel (XT). There’s no evidence
that administering an anthracycline after a taxane harms the patient
in any way. I eventually use an anthracycline; I just don’t
feel compelled to use it up front. The decision to use single-agent
taxane or single-agent capecitabine or the combination for frontline
therapy depends on factors such as the patient’s presentation
and the extent of her disease.
As we begin later-line therapy, when patients become more symptomatic
and heavily tumor-burdened, and their life expectancy is shortening,
a very reasonable argument can be made for better palliation and
maybe even better survival with a well-tolerated combination regimen.
Combination chemotherapy for late-line therapy
in patients with hormone refractory, HER2-negative disease
For late-line therapy in patients with hormone refractory, HER2-negative
disease, I prefer a well-tolerated combination. I love the combination
of capecitabine and vinorelbine. It’s the nonalopecia approach
to excellent palliative care, and I’ve used it often.
Combination therapy with chemotherapy and antiVEGFs
In the trial comparing capecitabine with or without bevacizumab
in patients with metastatic disease, the response rate was significantly
improved with the combination, but the primary endpoint did not
improve. It almost seemed like the VEGF antibody, bevacizumab,
had an initial antiangiogenic effect that increased the response
rates, which were not durable.
This suggests that perhaps, in late-line cancers, there are other
proangiogenic substances that may take over. It also suggests the
late-line patient population may not be the ideal place to study
angiogenesis inhibitors.
The ongoing randomized study of paclitaxel with or without bevacizumab
is an earlier-line trial in metastatic disease. Studies like this
need to be done because we can’t assume the response will
be the same in early- versus late-stage disease, and we may eventually
have to take it into the micrometastatic setting.
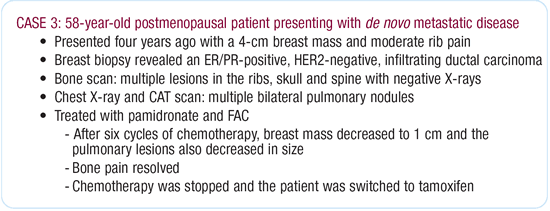
Dr O’Shaughnessy’s viewpoint
on the management of the primary lesion
I probably would not do anything further in terms of local therapy
to the breast at that point. I have a patient exactly like this
in my practice. She presented with bone and lung metastases but
had a locally advanced breast cancer and a mass in her other breast.
When we reduced the locally advanced tumor to a manageable size,
she had a salvage mastectomy because we were concerned it might
cause a local control problem.
The contralateral breast still has a small nodule, which we are
following closely; we will do a salvage mastectomy if it starts
growing. While I think surgeons would be inclined to excise the
lesion in this case, medical oncologists view it as just another
indicator lesion.
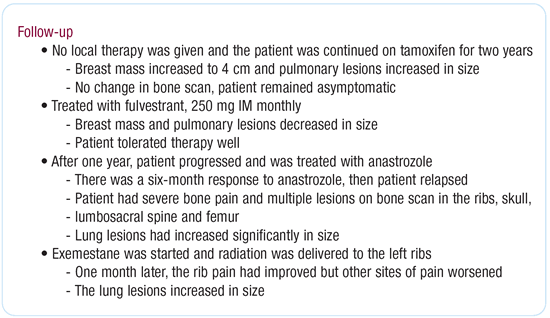
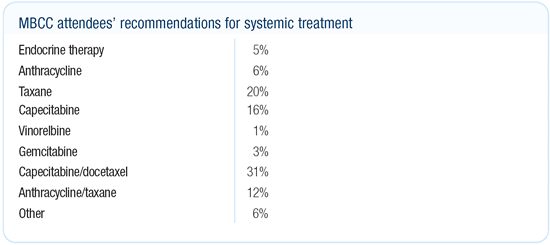
Dr O’Shaughnessy’s viewpoint
This patient could potentially be treated with a single cytotoxic
agent, and I believe the best choice would be either paclitaxel,
docetaxel or capecitabine. However, she’s very symptomatic
and better response rates are achieved with combination chemotherapy,
so I would treat her with capecitabine/docetaxel. If the combination
is well-tolerated, I continue administering it. If the combination
becomes too toxic, I discontinue docetaxel and utilize capecitabine
monotherapy.
The problem with combination therapy is you don’t know
whether the patient responded to the combination or one of the
single agents. I’ve had excellent responses with this regimen,
and the responses have been very durable on the continued capecitabine
monotherapy. If the patient then progress on singleagent capecitabine,
I usually stop the capecitabine and go to another therapy — either
single-agent vinorelbine or gemcitabine/carboplatin late-line.
Combination chemotherapy has a much higher response rate than
either single agent alone, but if the patient was relatively asymptomatic
and had a small volume of disease, you don’t really gain
anything by using the combination regimen. In these patients, giving
single agents sequentially is perfectly reasonable.
Select publications
|
