| You
are here: Home: BCU 6|2003: Robert
W Carlson, MD
Edited comments by Dr Carlson
Case discussion: 65-year-old woman with recurrent
ER-positive, HER2-negative breast cancer
History
This patient was initially diagnosed with breast cancer about
a decade ago. She had ER-positive, HER2-negative, node-positive
disease for which she received adjuvant CMF chemotherapy followed
by tamoxifen.
Several years following the completion of tamoxifen, she experienced
a biopsydocumented pulmonary recurrence. She had mediastinal lymphadenopathy
with some tracheal compression evidenced by both chest X-ray and
CT scan. She was active, although somewhat limited by dyspnea and
cough, especially upon exertion. Her Karnofsky performance status
was 70 to 80 percent. The symptoms had been evolving over several
months.
Since the recurrence was quite symptomatic, she was treated initially
with chemotherapy. We administered a three-hour paclitaxel infusion
every three weeks, and she had a good response.
Follow-up
She had symptomatic improvement quite rapidly in just four to
six weeks. Ultimately, we were able to document the improvement
radiographically, and we continued the taxane for about six months.
She had achieved a radiographic complete response and was experiencing
fatigue, alopecia and some neurotoxicity.
At that time her disease was not progressing. We discontinued
the taxane and started her on anastrozole, which was maintained
for a couple of years, and she continued to do well. Then she redeveloped
a pulmonary recurrence that was detected radiographically. Her
disease was persistently mediastinal, and she was having minimal
coughing at that time.
We elected to continue hormonal therapy and switched her to fulvestrant
in a single 5-mL injection. Her cough has improved, and serial
chest X-rays and CT scans have documented continued improvement
in her mediastinal disease. She has been on fulvestrant for almost
one year.
Discussion
If her disease progresses on fulvestrant and she doesn’t
have substantial endorgan dysfunction, I would consider another
endocrine maneuver; megestrol acetate or ethinyl estradiol would
be possibilities. Exemestane would also be a reasonable choice.
Exemestane’s toxicity profile is probably somewhat more acceptable
than either megestrol acetate or ethinyl estradiol. The crossover
rates of response from the nonsteroidal aromatase inhibitors to
exemestane are quite modest.
When we have exhausted all hormonal therapy options, sequential
single-agent vinorelbine or capecitabine would probably be my next
choice for her. I would likely choose capecitabine over vinorelbine,
but I think that either would be reasonable. Ultimately, she is
almost certainly going to be treated with both agents.
Patients prefer the convenience of oral therapy, and the response
rates for capecitabine are arguably equivalent to vinorelbine.
Most patients tolerate vinorelbine, but it requires weekly administration,
and a small number of women experience profound asthenia.
We do not start capecitabine at the FDA-approved dose. We typically
use capecitabine at 2,000 mg/m2 per day (total daily dose) divided
in two doses for two weeks on and one week off. Most women tolerate
that dose well for several cycles. The development of the hand-foot
syndrome is a problem that ultimately may require either a dose
reduction or prolongation of the one-week interval off therapy
to two or sometimes even three weeks.
Sequential single-agent versus combination chemotherapy
The available data support the use of either combination chemotherapy
or sequential single agents in patients with metastatic disease.
Typically, my practice is to use sequential single agents because
there is generally less toxicity and, arguably, equivalent efficacy.
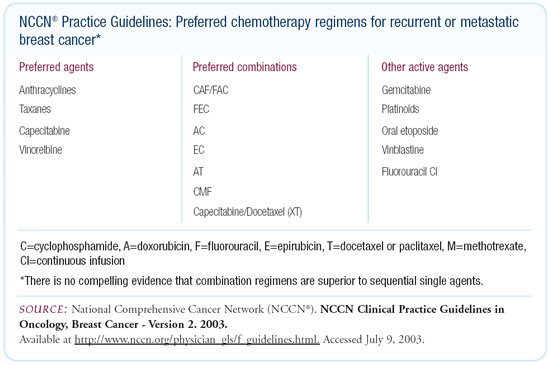
This is one situation in which the NCCN guidelines have changed
dramatically in the last year. Previously, the guidelines called
for combination chemotherapy in women with first relapse. They
recommended the use of either CMF or CAF or a single-agent taxane
and then, following failure, crossover to whatever had not been
given.
The current NCCN guidelines acknowledge the uncertainty that
combination chemotherapy is really superior. Now, we have a list
of preferred single agents. We have a list of preferred combinations,
and an acknowledgement that there is inadequate data to support
a dogmatic statement about whether sequential single agents or
combination chemotherapy should be used. We also list a series
of other active agents that could also be considered in the sequence.
It’s generally accepted that response rates are higher with
combination chemotherapy. The duration of ultimate disease control,
however, is not clearly superior for combination chemotherapy compared
to sequential single agents.
The patient who is more ill when beginning therapy may be less
able to tolerate aggressive combination chemotherapy; however,
that’s precisely the patient who needs aggressive combination
chemotherapy. I think it’s a situation in which clinical
judgment is important, and it’s crucial to involve the patient
in the decision-making process.
Chemotherapy followed by hormonal therapy
The practice of initially treating patients who have ER-positive
breast cancer with chemotherapy and then switching to hormonal
therapy is not addressed in the NCCN guidelines; however, it’s
a common strategy that makes sense.
In general, the NCCN guidelines classify women into two groups:
those who should be given endocrine therapy until they sequence
through all of them or develop organ impairment and those who should
be given chemotherapy until they have exhausted all of the reasonable
chemotherapy options.
The guidelines do, however, recommend that women who have substantial
organ dysfunction — even those with hormone receptor-positive
disease — be treated initially with cytotoxic chemotherapy.
Fulvestrant in ER/PR-positive, postmenopausal
patients with metastatic disease
Fulvestrant binds with the estrogen-receptor monomer in the cytoplasm
and prevents the dimerization of the estrogen receptor, which is
required for exertion of its maximal activity. Lack of estrogen-receptor
dimerization results in accelerated degradation of the ER-fulvestrant
complex. Ultimately, there is a loss of estrogen receptors within
the cells.
The estrogen receptor is continually regenerated, so continued
exposure to fulvestrant is required. After fulvestrant is discontinued,
the estrogen receptor will, with time, reappear in cells. The fact
that we see subsequent hormonal responses is convincing biological
or clinical evidence that the estrogen receptors do reappear.
Injection site reactions and hot flashes are the only side effects
that I’ve observed in patients receiving fulvestrant. There
may be something about the administration technique for fulvestrant
that can affect the pain that is infrequently experienced. If the
injection is inadvertently given subcutaneously into fat, it’s
more painful than if it’s given intramuscularly. It may be
that many of the women who have pain with the injection are not
actually receiving true intramuscular injections; this is more
likely to occur in women who are obese.
Sequencing of hormonal therapies
Women with breast cancer who fail on tamoxifen can clearly respond
to fulvestrant, and the rate of response is equivalent to that
seen with anastrozole. Also, in women with disease that has failed
anastrozole who are then crossed over to fulvestrant, the rate
of clinical benefit is substantial and in the range of about 40
percent. Patients who are crossed over from fulvestrant to aromatase
inhibitors also show response rates around 40 percent.
Surprisingly, the magnitude of benefit from fulvestrant does
not predict whether the cancer will respond to a subsequent hormonal
maneuver. One rule of thumb in the past has been that the magnitude
and duration of response to the most recent hormonal therapy predicts
for the likelihood of response for subsequent hormonal therapies.
A small retrospective study suggests that may not be the case with
fulvestrant.
Novel hormonal therapy combinations
There is an increasing body of preclinical evidence suggesting
that breast cancers that become resistant to tamoxifen or fulvestrant
have upregulation of epidermal growth factor receptor (EGFR) and
HER2 expression. As those endocrine-sensitive cells become endocrine-resistant
and the EGFR and HER2 upregulate, some of the sensitivity to the
endocrine agents may return if those cells are exposed to EGFR
inhibitors.
There are series of trials being conducted to evaluate the role
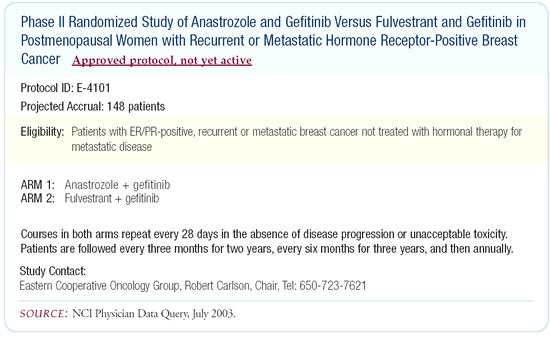
of fulvestrant or other hormonal agents in combination with gefitinib.
ECOG is initiating a Phase II randomized trial comparing fulvestrant/gefitinib
to anastrozole/gefitinib.
Trastuzumab in combination with fulvestrant would be a very interesting
study. There is evidence that HER2-overexpressing tumors are relatively
more hormoneresistant. There’s a lot of cross talk between
those pathways, and that study would be similar to the studies
looking at gefitinib plus fulvestrant. We can extend that thinking
even further and look at the utilization of hormonal therapies
in combination with an EGFR inhibitor and a HER2 inhibitor.
The combination of an aromatase inhibitor and fulvestrant is
of some interest, but the difficulty with such a study is that
fulvestrant eliminates the estrogen receptor. Theoretically, if
the estrogen receptor is eliminated, then the cells shouldn’t
care how much estrogen is present.
ATAC trial results
Many of us were surprised that anastrozole alone was superior
to tamoxifen in the ATAC trial. The difference in contralateral
breast cancers was remarkable, with a 75 percent risk reduction
for anastrozole compared to what we would expect with placebo.
I’m not totally surprised that the differences were seen
so soon; presumably most of the breast cancers prevented from being
diagnosed in those women were pre-existing breast cancers. Relatively
few women in the ATAC trial actually received cytotoxic chemotherapy,
so the contralateral breast cancers should have already been present.
The ATAC trial is an important trial biologically, demonstrating
that the aromatase inhibitors likely have a very important role
in early breast cancer. The differences seen between tamoxifen
and anastrozole, especially if they’re maintained with further
follow-up, are substantial and clinically significant.
Implications of the ATAC trial on clinical practice
As a result of the ATAC trial, my practice pattern changed overnight.
I am not treating all of my patients with anastrozole, but I am
certainly discussing the results of the ATAC trial and the pros
and cons for tamoxifen and anastrozole. I’m using shared
decision-making with patients to determine which of the agents
they prefer. The recent update at the San Antonio meeting in December
2002 confirmed that practice.
Generally, I recommend anastrozole; however, there are other
factors to consider that would sway me one way or another. Obviously,
in women with an absolute or relative contraindication to tamoxifen,
it’s a very easy decision. Conversely, there are patients
who may have relative contraindications to anastrozole.
The major relative contraindication is severe osteoporosis. The
bone mineral density loss associated with the aromatase inhibitors
is a concern. Presumably, we can blunt that effect using bisphosphonates,
so it is unlikely to be a major problem.
The patient’s nodal status does not make a great deal of
difference to me in terms of hormonal therapy recommendations.
I look at the patient’s HER2 status, and it does shade my
thinking a bit. There is some data, although somewhat contradictory,
that HER2-overexpressing tumors may be relatively resistant to
tamoxifen.
Likewise, there is data suggesting that both letrozole and anastrozole
maintain antitumor activity in HER2-overexpressing tumors. I think
it would be reasonable to consider anastrozole, in preference to
tamoxifen, for patients with tumors that have an IHC score of 2+
or 3+.
NCCN Practice Guidelines: Adjuvant hormonal
therapy
In response to the initial presentation of the ATAC results,
the NCCN guidelines were modified. Tamoxifen was maintained as
the recommended adjuvant therapy in the text of the guidelines.
There is, however, a footnote to the guidelines stating that anastrozole
should be considered as an alternative to tamoxifen. The guidelines
recommend a discussion between the physician and the patient regarding
tamoxifen and anastrozole as adjuvant therapy.
The guidelines state that anastrozole may, in fact, be superior
to tamoxifen, but we need to recognize there is short follow-up
with adjuvant anastrozole relative to very long follow-up with
tamoxifen. Because of that, it’s difficult to be dogmatic.
To some extent, it depends on the woman — is she someone
who is an early adaptor of a new therapy, or is she someone who
is more conservative in terms of adopting new technology or new
therapies?

ASCO Technology Assessment regarding adjuvant
aromatase inhibitors
The ASCO Technology Assessment is a superb document, but it needs
to be viewed for exactly what it is. A technology assessment looks
at a given therapy, attempts to decide whether that therapy has
utility in a given clinical situation and determines what the preponderance
of data is within that clinical situation. The ASCO Technology
Assessment, in both the first and second versions, states that
tamoxifen remains the standard adjuvant therapy to which other
therapies should be compared.
Interestingly, several members of the ASCO Technology Panel also
sit on the NCCN Practice Guidelines Panel. When the NCCN Practice
Guidelines Panel looked at this issue, there was no major dissension
in considering anastrozole as an option. The difference between
groups occurred because of the different processes.
The ASCO Technology Assessment is strictly evidence-based, and
it cannot go beyond the evidence. So, there are no extrapolations
beyond five years of anastrozole or the 47 months of follow-up.
In the NCCN Practice Guidelines process, we use a methodology
called evidence-based consensus. We establish recommendations based
on evidence, but we are also able to use expert consensus in situations
where the evidence is lacking. Obviously, 10-year data with adjuvant
anastrozole are lacking, but we can come up with expectations about
what might happen and make recommendations that extrapolate into
the unknown.
The NCCN Practice Guidelines are patient-focused, and they look
at the various therapies that are available from a patient’s
perspective. The NCCN Guidelines Panel believes that women should
consider the use of anastrozole, although we don’t say it
should necessarily be used in preference to tamoxifen.
Nonprotocol use of adjuvant letrozole or exemestane
I’m not using either letrozole or exemestane in the adjuvant,
nonprotocol setting, primarily because all the data we have is
with anastrozole. I await, with a great deal of interest, the results
of the many ongoing adjuvant trials evaluating letrozole and exemestane.
Until we have that data, I think it’s premature to consider
they are equal or even superior to anastrozole in the adjuvant
setting.
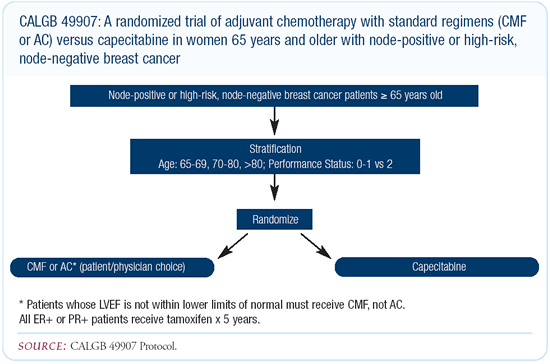
CALGB-49907: Adjuvant chemotherapy trial in
elderly women
I anticipate we will participate in Hyman Muss’ trial comparing
capecitabine to CA or CMF in the adjuvant setting. It’s an
excellent study that will compare a well-tolerated single agent
to two generally well-tolerated combinations.
The difficulty with that study is two-fold. First, the magnitude
of benefit from cytotoxic chemotherapy in older women is not clear,
and if there are benefits, they are likely to be small. Second,
it’s going to be very difficult to detect differences between
the combination arm and the single-agent arm if, in fact, they
exist, because they are likely to be so small.
There is likely to be a significant difference in quality of
life, side effects and tolerability for the three regimens. Which
of those regimens — AC or CMF or single-agent capecitabine — will
ultimately be better tolerated is relatively unpredictable. The
hand-foot syndrome associated with capecitabine is a problem for
many women, and whether they will find it more acceptable than
the nausea, vomiting, alopecia, etc., associated with CMF and AC,
we’ll have to wait and see.
All women are concerned about alopecia as a side effect, but
many women would find alopecia an acceptable side effect for the
benefits associated with cytotoxic chemotherapy. However, I’m
equally confident that no woman looks forward to experiencing alopecia.
We underestimate how much of an issue alopecia is for women; it
is very commonly the most feared side effect.
Women who have nausea and vomiting realize it’s something
they can experience in the privacy of their home. The same applies
to myelosuppression; women may have problems with fever and neutropenia,
but when they walk out of their home nobody realizes they have
myelosuppression. Women who have alopecia feel very uncomfortable
when they walk outside their home. Many are quite uncomfortable
with their wigs and don’t feel they look good. Alopecia is
the toxicity women complain to me about the most.
Oral therapy is always advantageous for the compliant patient.
One of the difficulties is assuring that the patients are compliant
with their therapy. The oral regimens are especially beneficial
in situations where accessibility to a medical oncologist is limited — in
rural and underserved communities.
Adjuvant dose-dense chemotherapy
My expectation is that we will see dose-dense chemotherapy added
to the NCCN Practice Guidelines. The real question is: How will
it be weighted within the guidelines? If one looks at the duration
of follow-up and magnitude of risk reduction with dose-dense chemotherapy
compared to what is achieved with anastrozole, they are almost
superimposable.
I have started using dose-dense therapy in selected patients.
As we translate the results of dose-dense therapy into practice,
one needs to be careful about who is selected for such therapies.
Initially, I tend to use it more in the younger, highrisk patient.
As I become more familiar with it, my indications will expand.
We also need be cautious not to jump to the conclusion that dose-dense
therapy will be superior when long-term data are available. I’m
hopeful that it will be, I expect it to be, but we’re going
to have to continually look at that data.
Select publications
|
