| You
are here: Home: BCU 6|2003: Monica
Morrow, MD
Edited comments by Dr Morrow
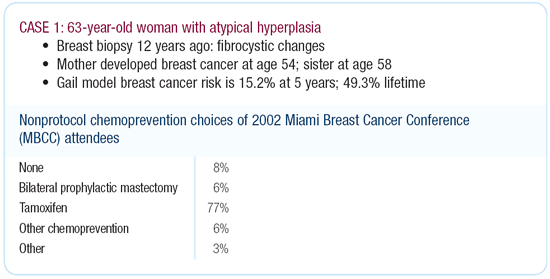
Dr Morrow’s viewpoint
Tamoxifen would be a good option for this woman based on her
high-risk profile. Interestingly, the data we generated from our
own practice clearly shows that women who are at risk on the basis
of histologic lesions — atypical hyperplasia and LCIS — are
far more likely to be offered and to accept tamoxifen than women
with equivalent levels of risk due to other factors. Approximately
60 to 65 percent of my patients with atypia take tamoxifen. In
general, only about 25 percent of women at high risk are offered
tamoxifen and accept.
Genetic counseling should be considered for this patient. I would
take a more detailed family history to determine how many relatives
were affected and if she is of Ashkenazi descent. Her risk might
be substantially higher than the Gail model suggests, and if knowing
that would change the way she manages her risk, then genetic counseling
would be beneficial.
Most women do not want a prophylactic mastectomy, but if a patient
tells me her level of risk is unacceptable and she wants to maximally
reduce her risk, then it’s an option. It’s important
that the patient fully understands her risk, the sequelae of surgery,
the possible complications and the other available options to reduce
risk, such as tamoxifen or, in gene carriers, oophorectomy.
I also counsel these patients that should they develop breast
cancer, the chances are 80 to 90 percent that they would be treated
with a breast-conserving approach rather than mastectomy. It’s
difficult for me to understand why women with a breast cancer risk
of less than five or ten percent would opt for such a radical approach
as prophylactic mastectomy. In these women with low risk, education
is particularly important because they have often been told they
need this surgery because their breasts are dense and lumpy.
Studies have clearly shown that women interested in prophylactic
mastectomy tend to overestimate their level of risk by approximately
10-fold, and it takes a lot of time to get past that fear. For
the woman who understands that her risk is low but seems intent
on prophylactic mastectomy, psychological counseling should be
employed to determine what is driving that decision.

Dr Morrow’s viewpoint
The STAR trial would be an excellent option for this woman. It
is designed to show whether tamoxifen or raloxifene is the better
preventive agent, and it also evaluates side effects and the impact
of each drug on overall health. The relatively low bioavailability
of raloxifene raises concern that it may not be the ideal drug,
particularly in younger, postmenopausal women. Raloxifene is currently
being studied to see if it reduces the risk of coronary heart disease
and, from a compliance perspective, this is important because it
will probably be easier to convince women to take a drug with multiple
health benefits than one that’s purely a breast cancer preventive.
“The Raloxifene Use for The Heart (RUTH) trial is an
international, multicenter, randomized,
double blind, placebo-controlled trial designed to evaluate
whether 60 mg/day of oral
raloxifene compared with placebo reduces the risk of coronary
events (coronary death, nonfatal
myocardial infarction [MI], or hospitalized acute coronary
syndromes other than MI) and risk of
invasive breast cancer in postmenopausal women with documented
coronary heart disease
(CHD) or who are at increased risk for major coronary events.” |
 |
SOURCE: Wenger NK. Baseline
Characteristics of Participants in the Raloxifene Use for
The
Heart (RUTH) Trial. Am J Cardiol 2002;90:1204-10.
Abstract |
Dr Morrow’s viewpoint
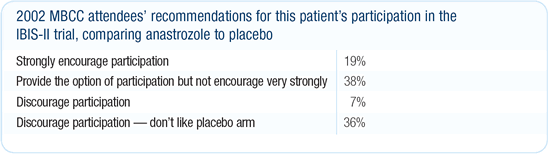
The research question about aromatase inhibitors as preventive
agents is a very important one, but I am concerned that the IBIS-II
trial won’t give us the answer we need. We’ll know
if anastrozole is better than a placebo but we won’t know
how SERMs compare to aromatase inhibitors or which is better in
terms of overall health. We will not be able to extrapolate these
answers from two completely different study populations, and this
will leave us with another trial to do. I would not recommend IBIS-II
to this patient with atypical hyperplasia or any woman at high
risk. I don’t think taking a 50 percent chance of being randomized
to a placebo is a good choice. In addition, if the osteoporosis
and fracture rates seen in the current aromatase inhibitor treatment
trials persist, we will have another set of issues to address.
Telling women they’ll just have to take another drug to
protect against osteoporosis while they’re taking an aromatase
inhibitor to protect them against breast cancer is problematic.
Aside from the highest-risk or very motivated patients, how many
women are going to take multiple pills to prevent something for
which they’re not having any symptoms?
IBIS-II also has a randomization for women with DCIS, which compares
anastrozole to tamoxifen. I agree that treating DCIS is primarily
prevention — it’s a lesion that carries a significantly
increased risk of invasive breast cancer. We tend to think of it
differently because we treat it like cancer, but the question is
the same. The NSABP-B-35 trial is asking the same question, randomizing
women with DCIS to anastrozole versus tamoxifen. It is a good trial,
addressing an important question, and I heartily support that study.
| “Anastrozole will also be tested in the upcoming NSABP
Trial B-35... .Eligible subjects will be postmenopausal women
with DCIS who are treated with lumpectomy and radiation therapy.
They will be randomly assigned to treatment with either tamoxifen
(20 mg daily) for 5 years or anastrozole (1 mg daily) for 5
years. The design and measured outcomes of the trial will be
similar to those in NSABP Trial B-24: the occurrence of invasive
breast cancer in either the ipsilateral or the contralateral
breast, the occurrence of DCIS in the contralateral breast,
and the recurrence of DCIS in the ipsilateral breast, as well
as local, regional, and distant event rates.” |
 |
| SOURCE: Vogel VG et al. National
Surgical Adjuvant Breast and Bowel Project Update: Prevention
Trials and Endocrine Therapy of Ductal Carcinoma in Situ. Clin
Cancer Res 2003;9:495s-501s. Abstract |
Dr Morrow’s viewpoint
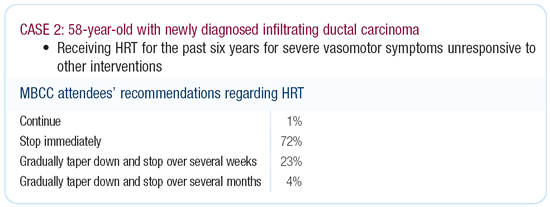
I think everyone agrees that patients like this should stop hormone
replacement therapy. The real question is: Do they have to stop
it immediately and be miserable while you’re preparing them
for surgery or can they taper down? I have become quite comfortable
with tapering patients over a month or so.
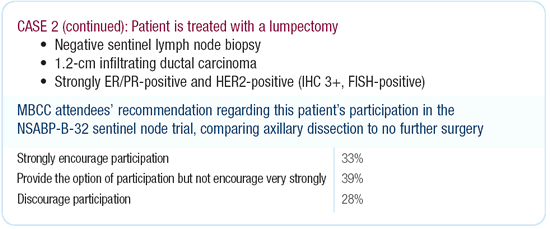
Dr Morrow’s viewpoint
I think the primary question asked by NSABP trial B-32, namely,
whether removing negative lymph nodes improves survival, was answered
by the NSABP approximately 25 years ago in B-04, so this is not
my favorite trial. It’s excellent for physicians learning
the technique of sentinel node, but I don’t believe it would
benefit this patient.
“The underlying hypothesis to be tested in this trial
[NSABP-B-32] states that patients who
have pathologically negative SLNs will have equivalent disease-free
and overall survival rates, if
they are treated by sentinel node biopsy alone or sentinel
node biopsy plus completion axillary
dissection. A second part of this hypothesis is that the morbidity
of the sentinel node biopsy
alone will be significantly less than that of the sentinel
node plus axillary dissection, therefore
tipping the scales favoring the sentinel node biopsy procedure.” |
 |
SOURCE: Harlow SP, Krag DN. Sentinel
lymph node—Why study it: Implications of the B-32
study. Sem Surg Oncol 2001;20:224–229. Abstract |
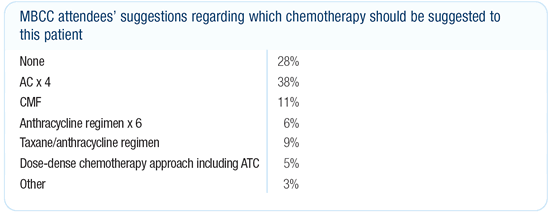
Dr Morrow’s viewpoint
With a 1.2-centimeter, node-negative, estrogen receptor-positive
cancer, the amount of additional benefit in terms of absolute gain
from chemotherapy is very small. I would not encourage this patient
to have chemotherapy for what I would estimate to be a one or two
percent survival benefit. However, I think most medical oncologists
in the United States would recommend chemotherapy followed by endocrine
therapy in this case.
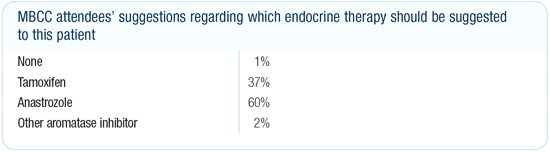
Dr Morrow’s viewpoint
This patient will derive the greatest benefit from endocrine therapy.
For women with low-risk, ER-positive, HER2-negative breast cancers
with very favorable prognosis, we still use as much tamoxifen as
anastrozole. For women with HER2-positive breast cancers, as in
this case, I favor an aromatase inhibitor because of the debate
about whether HER2 overexpression predicts resistance to tamoxifen.
The follow-up data with anastrozole from the ATAC trial are encouraging
and suggest that the bone problems may be reaching a plateau.
If the patient’s prognosis was less favorable, I would
be more likely to treat her with an aromatase inhibitor, regardless
of the tumor HER2 status. There is clearly a greater benefit from
anastrozole compared to tamoxifen in the short term. In a patient
whose risk of relapse is quite high, the absolute difference between
these two treatments is much larger. I would favor an aromatase
inhibitor in the high-risk setting, and the data we have right
now in the adjuvant setting is with anastrozole.
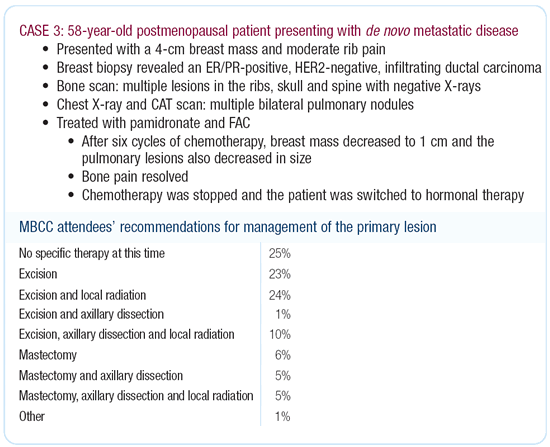
Dr Morrow’s viewpoint
I have traditionally thought that in this situation we should
only treat the primary tumor if it was progressing and causing
local problems; however, last year my colleague, Seema Kahn, and
I published a study in the Journal, Surgery, of over 15,000 women
from the National Cancer Database of the American College of Surgeons
who presented with metastatic disease. This was based on tumor
registry data.
We looked at differences in survival based on surgical treatment
of the primary lesion versus no surgery. We controlled for number
of documented metastatic sites and visceral versus soft tissue
disease, and we found a very consistent pattern wherein surgical
treatment of the primary lesion was associated with improved survival.
While there may be selection bias to some extent, the differences
were seen in all subgroups.
This study raises some questions as we develop more effective
systemic therapy and keep people alive longer. Does it make sense
to reduce the tumor burden maximally, so there are fewer places
the treatment has to work? We see this in renal cell carcinoma,
for example, where removal of the primary tumor results in a survival
advantage. I think it’s an open question. However, removal
of the primary lesion is a reasonable option in this patient to
try to maintain local control and prevent morbidity, even if it
doesn’t improve survival. If the patient is clinically node-negative,
I don’t see that there’s a lot to be gained by dissecting
the axilla.
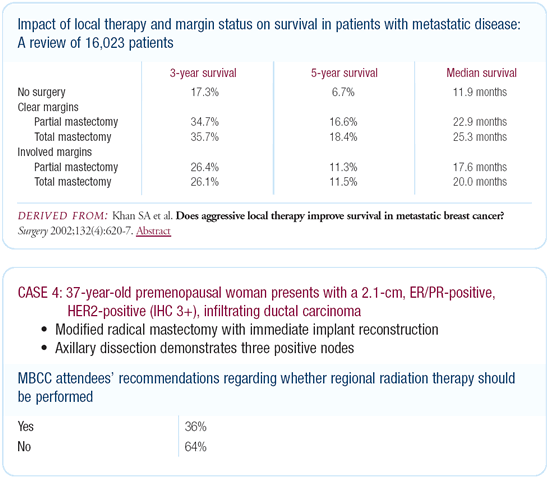
Dr Morrow’s viewpoint
The risk of local relapse on the chest wall is certainly high
enough to warrant radiation. There’s nothing magical about
three versus four positive nodes — it’s a continuum.
To an extent, the decision to irradiate depends on the characteristics
of the metastases. Gross disease in the nodes, extranodal extension,
lymphatic invasion at the primary site — features such as
these would push me in the direction of radiation. Data suggest
younger women have a higher risk of chest wall relapse after mastectomy,
just as they have a higher risk of local failure after lumpectomy.
Putting all these factors together, I would certainly discuss radiation
with this patient.
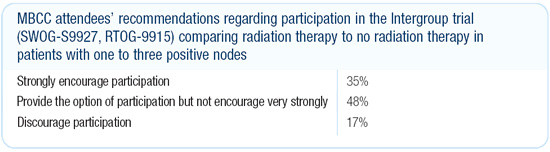
Dr Morrow’s viewpoint
We participate in this study and I think it’s a trial that
needs to be completed, but it’s not accruing well. One reason
may be that — in the medical oncology community — the
idea that radiotherapy contributes to survival is heresy, and it’s
the medical oncologist who would refer these patients after their
systemic therapy to radiotherapy where they would hear about the
trial.
(Editor’s Note: Subsequent to this interview, RTOG-9915
was closed due to poor accrual.)

Dr Morrow’s viewpoint
We would definitely not remove the expander. Our approach to
reconstruction has evolved as the indications for postmastectomy
radiotherapy have increased. In women who have a high likelihood
of needing radiation therapy after surgery, we put in an expander
to allow us to save the skin and do a small skinsparing type of
incision. These are generally patients who will also require a
more prolonged course of chemotherapy and the expander gives them
a breast mound. If they are satisfied with the cosmetic results
afterward, they’re done. If they’re not satisfied,
then they can undergo tram flap reconstruction.
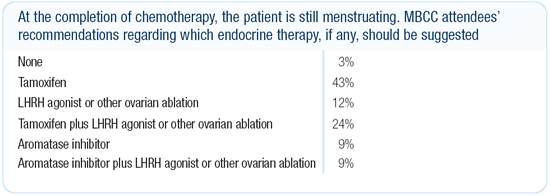
Dr Morrow’s viewpoint
While there is no definitive evidence, there are data suggesting
that ovarian suppression improves outcome in premenopausal patients
with ER-positive breast cancer. Therefore, in premenopausal women
with a poor prognosis, we include ovarian suppression in our treatment
plan. We need clinical trials to look at the combination of ovarian
suppression plus aromatase inhibitors versus ovarian suppression
plus tamoxifen. That is an important comparison, and it will inform
us how important the estradiol elevations are in premenopausal
women receiving tamoxifen.
The significance of micrometastatic disease
in axillary nodes
The increasing use of sentinel node biopsy has raised a whole
new set of questions including whether micrometastases detected
by immunohistochemistry are clinically significant. This is a biologically
interesting question, and I strongly agree with the College of
American Pathologists’ consensus statement that we do not
yet understand the meaning of these micrometastases.
The retrospective studies of micrometastases have been a “mixed
bag,” including patients who have large areas of missed tumor
in their lymph nodes and patients with small numbers of cells in
subcapsular sinuses that aren’t even in the node parenchyma.
It’s not particularly surprising that some of these studies
show no survival difference, some show small survival differences,
and others show very big survival differences.
This is an area where both the NSABP-B-32 sentinel node study
and the American College of Surgeons Z-10 study will provide us
with very important information. Until that information is available,
we use immunohistochemistry only if there’s diagnostic uncertainty
on the basis of something seen on an H&E stain. We do not routinely
perform immunohistochemical staining of sentinel lymph nodes because
we don’t know what to tell the patients.
Neoadjuvant therapy
One of the more interesting observations about neoadjuvant therapy
is from the recent NSABP-B-27 trial, which demonstrated that you
can drive clinical and pathologic responses by adding a taxane,
but the breast conservation rate is not increased. Currently, in
the absence of a proven survival benefit for preoperative therapy,
the only reason to give neoadjuvant therapy is to increase the
rate of breast conservation.
We reserve neoadjuvant therapy for the patients who want breast
conservation but have tumors that are too large to allow it. Surgery
following the downsizing of such a tumor is clearly different than
a primary lumpectomy. If we resect a smaller volume of the breast
than was originally occupied by the tumor and there’s viable
tumor scattered all around the specimen, even if the margins are
negative, we have to be concerned there may be tumor left in the
breast and we resect again. If that’s negative, then we’re
satisfied, but if there’s viable tumor in that re-resection,
then we rethink whether breast conservation is appropriate. The
NSABP study showed that the local failure rate in women downstaged
by chemotherapy for breast conservation was twice as high as the
rate in women who originally were candidates for breast-conserving
therapy.
Underutilization of breast-conserving surgery
in the United States
The NSABP-B-06 trial began when I was a surgical resident, and
at that time there were violent arguments over radical versus modified
radical mastectomy. We see that carryover today. There is probably
no surgical operation that has undergone as much intense scientific
scrutiny as breast conservation, and yet a substantial number of
women with Stage I and II breast cancer are still being treated
with mastectomy in this country.
Clearly, some physicians have not gotten past the notion that
mastectomy is better, and they convey that to patients. In our
study of second opinions, we found that even among educated, insured
women with access to the best health care, fewer than one-half
of them had been advised that there are three surgical options
for the treatment of breast cancer.
Still, there is clearly a population of women who prefer mastectomy,
and it’s difficult to know whether that’s because of
an unreasonable fear of local recurrence or because they want to
avoid radiation.
In our experience, younger women choose breast conservation at
the same rate as older women. The primary predictors for who will
choose mastectomy are women who have Medicare or Medicaid and live
in the South or the Midwest part of the country. Also, bad prognostic
cancer features correlate with a greater likelihood of having a
mastectomy, even though they have nothing to do with that choice.
Select publications
|
