| You are here: Home: BCU 9|2003: Stephen E Jones, MD
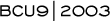
Edited comments by Dr Jones
Phase III randomized trial (Taxotere-311) comparing docetaxel to paclitaxel in patients with metastatic breast cancer
The Taxotere-311 trial started in 1993 and was completed in 2003. Peter Ravdin, the principal investigator, presented some of the data at the ECCO meeting in September 2003, and I presented the data at the 2003 San Antonio Breast Cancer Symposium.
With the approval of docetaxel, the FDA mandated a trial comparing docetaxel 100 mg/m2 to paclitaxel 175 mg/m2, each administered on an every-three-week schedule. Over 400 patients with relatively anthracycline-resistant disease were accrued; they had either relapsed on an anthracycline-containing regimen or within 12 months of receiving one.
The results were a bit surprising, and I didn't think they would be quite so dramatic. For the evaluable patients, there was a significant difference in the response rate, time to tumor progression and survival in favor of docetaxel. There was more toxicity associated with docetaxel than with paclitaxel, but it was the usual manageable toxicity.
This study basically confirmed that docetaxel was probably a more potent taxane, at least on an every-three-week schedule. The survival advantage was surprising. In fact, there aren't many regimens with a documented survival advantage in patients with metastatic breast cancer.
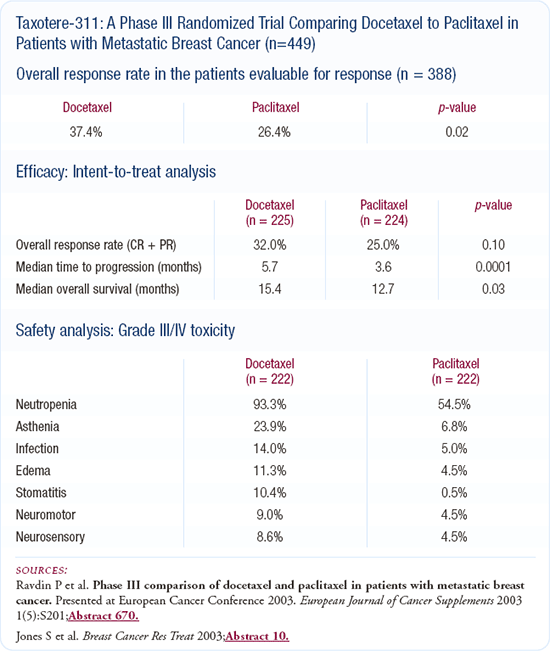
Obviously, adding trastuzumab to paclitaxel or to an anthracycline has a survival advantage relative to chemotherapy alone. Joyce O'Shaughnessy's trial, comparing capecitabine and docetaxel to docetaxel alone, also demonstrated a survival advantage.
Sequential single-agent versus combination chemotherapy in patients with metastatic breast cancer
The big question associated with the sequential single-agent versus combination chemotherapy trials is the effect of crossover therapy. In Joyce O'Shaughnessy's trial, we don't know what the effect on survival would have been if 60 or 70 percent of the patients treated with single-agent docetaxel were then treated with capecitabine. Maybe there would not have been a survival difference. Hence, the effect of crossover therapy remains a question in all of these trials comparing doublets to single-agent regimens.
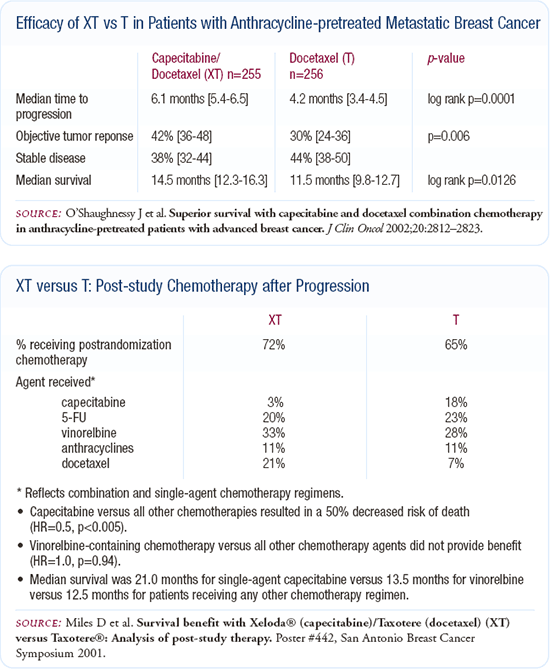
I generally prefer single-agent chemotherapy, but I discuss combination chemotherapy with my patients and offer them a choice. In clinical practice, my approach has been to use combination chemotherapy when I can't wait for a response.
In the patient with limited disease who needs chemotherapy, in whom I'm hoping to obtain a complete remission, consolidate the sites of disease with radiation or if there is a chance for a prolonged remission, I would probably also favor combination chemotherapy. If the treatment is strictly for palliation or to try to control the cancer, I'm probably going to use sequential single-agent chemotherapy.
US Oncology adjuvant capecitabine and docetaxel (XT) trial
NSABP conducted a neoadjuvant trial (NSABP-B-27) comparing four courses of AC to four courses of AC followed by docetaxel. In that trial, the addition of docetaxel doubled the pathologic complete response rate. Therefore, our Breast Committee at US Oncology has now assumed that four courses of AC followed by docetaxel is the standard treatment. In our adjuvant XT trial, patients will be randomly assigned to AC followed by docetaxel or AC followed by docetaxel and capecitabine.
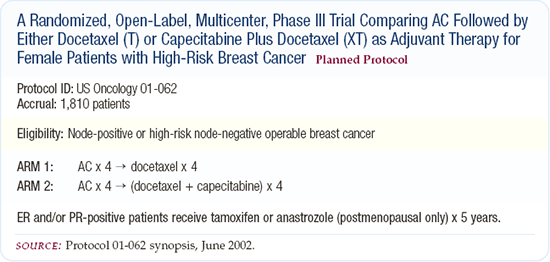
US Oncology adjuvant docetaxel and cyclophosphamide trial
At ASCO 2003, I presented the first planned analysis of an adjuvant trial comparing four cycles of docetaxel and cyclophosphamide (TC) to four cycles of doxorubicin and cyclophosphamide (AC). The trial was underpowered with 1,016 total patients and approximately 500 patients per treatment arm. The patients were pre- or postmenopausal and had either node-negative or node-positive disease. At 42 months of follow-up, there were fewer recurrences in the patients treated with TC than those treated with AC.
We had previously demonstrated that TC was a little better-tolerated than standard AC. Patients treated with TC had some of usual docetaxel-related side effects (e.g., arthralgias, peripheral neuropathy, etc.), but they had less mucositis, anemia, nausea and vomiting.
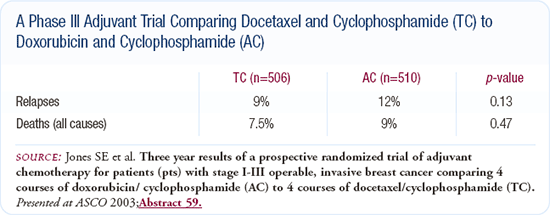
I use adjuvant TC for patients as an alternative to anthracycline-based regimens. I see little reason to use CMF, and I've used TC in patients with heart disease or those previously treated with doxorubicin. The TC regimen has no cardiac toxicity or long-term toxicities at 42 months.
For many patients with node-negative disease, four cycles of adjuvant AC is standard treatment, but if there were any hesitancy to use it because of heart disease or other issues, I would use four cycles of TC.
Recent adjuvant chemotherapy trials in patients with node-positive disease
We now have a number of adjuvant regimens that are better than the standard regimens. I'm intrigued by the dose-dense approach, but before I adopt it routinely, I want to see confirmation from a second trial. Two trials evaluating AC followed by paclitaxel have reported a significant improvement with that adjuvant regimen.
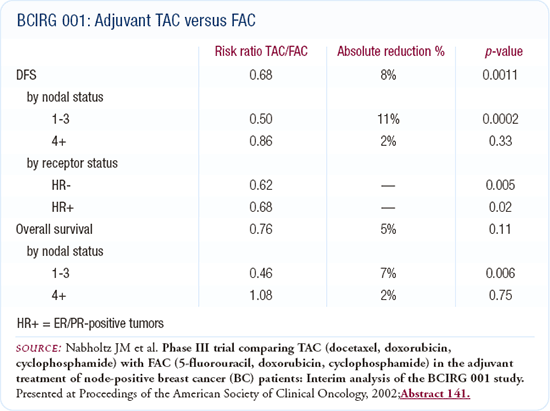
The NSABP-B-28 trial, which added four cycles of paclitaxel to AC, had results similar to the earlier study. Many oncologists have substituted docetaxel for paclitaxel, and the Taxotere-311 data lends support to that in the adjuvant setting. In a younger patient with node-positive disease who is not eligible for a trial, I am more likely use AC followed by docetaxel.
The study comparing docetaxel, doxorubicin and cyclophosphamide (TAC) to 5-fluorouracil, doxorubicin and cyclophosphamide (FAC) is a very clean trial. It is often interpreted as TAC being more effective for patients with one to three positive nodes, but not those with four positive nodes. However, that is the way the data were presented, and TAC is pretty effective across the board. Some oncologists have expressed concern about the TAC regimen's toxicity, and it probably requires the use of growth factors.

Fulvestrant in patients with metastatic breast cancer
Fulvestrant has a different mechanism of action than the other hormonal agents because it downregulates both the estrogen and progesterone receptors. It's a well-tolerated parenteral agent - a potential advantage for patients with compliance issues. There is a subset of patients who had an exceptionally long duration of response with fulvestrant, and this is not fully appreciated.
US Oncology participated in one of the trials comparing fulvestrant to anastrozole, and I personally enrolled 27 patients in the study. Five of those patients had responses lasting longer than three years, which is really extraordinary for any endocrine treatment; two of the patients had responses lasting longer than four years. Of those five patients, four have progressed and had their therapy unblinded; all four were on fulvestrant. I would bet the fifth patient, although her treatment remains blinded, is also on fulvestrant.
A reanalysis of the North American and the European fulvestrant trials used a different statistical model called the mean duration of response. In that statistical model, values were assigned to every patient: patients with disease that did not respond were assigned a value of zero and patients with disease that did respond were assigned a number to correspond with the number of months of the response. With those calculations, fulvestrant had a significantly longer duration of response. It was 36 percent longer in the North American trial and 27 percent longer in the European trial.
In this country, I see fulvestrant being used as a third- or fourth-line hormonal therapy; however, studies indicate that it might be better than anastrozole following disease progression on tamoxifen. I encourage physicians who are going to try fulvestrant to use it in women progressing on tamoxifen.
The paradigm will probably shift because more patients will be treated with adjuvant anastrozole. We don't know where fulvestrant will fit into that sequence in a patient who has never received tamoxifen whose disease relapses after adjuvant anastrozole.
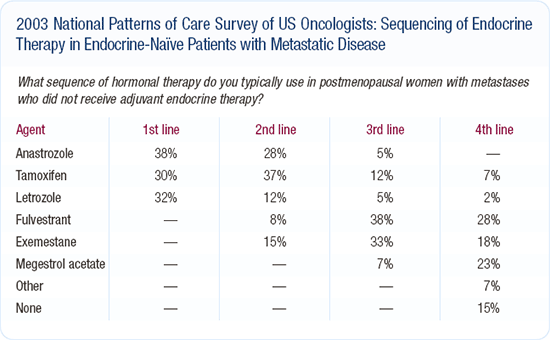
Sequencing hormonal agents in postmenopausal women
In a postmenopausal woman whose disease relapses on adjuvant tamoxifen, I would use fulvestrant because I've seen some very long remissions with it. I will use an aromatase inhibitor later because data indicate that patients with disease that progresses on fulvestrant can still respond to other endocrine treatments (e.g., aromatase inhibitors and megestrol acetate).
A couple of reports have looked at the response to fulvestrant in patients who have received an aromatase inhibitor. A fairly small Swiss study reported that about one-third of patients derived clinical benefit from fulvestrant after treatment with tamoxifen or an aromatase inhibitor. A compassionate-use study, reported at ASCO 2003, reported about 60 patients with fulvestrant as second-, third- or fourth-line therapy. Fulvestrant had a more than 50 percent clinical benefit rate in those patients.
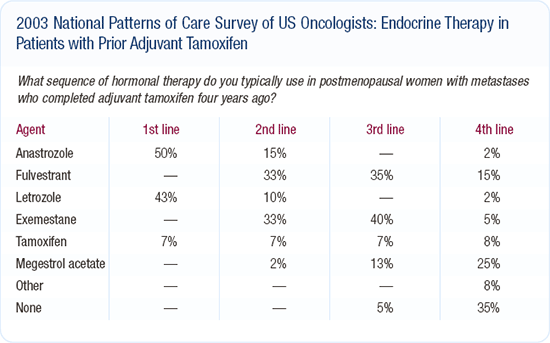
Adjuvant hormonal therapy for postmenopausal women
The ATAC trial has had a major impact across the country, and we are seeing more adjuvant anastrozole being used. The ATAC trial results must be discussed with patients, and patients should be aware of the two hormonal therapy options. Many factors go into making a decision about hormonal therapy, including the patient's ability to pay for the drug, her feelings and her history of thromboembolic events.
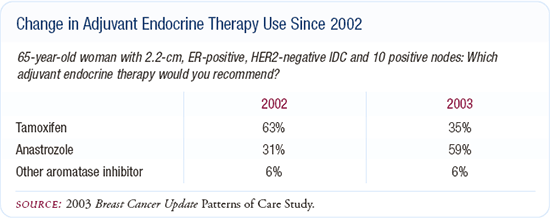
I am more likely to use adjuvant anastrozole in the patient with higher-risk, node-positive disease. The woman with 10 positive nodes needs every percentage point possible to make sure her cancer doesn't recur. In that type of patient, I would try to encourage patients to receive anastrozole.
Adjuvant trastuzumab
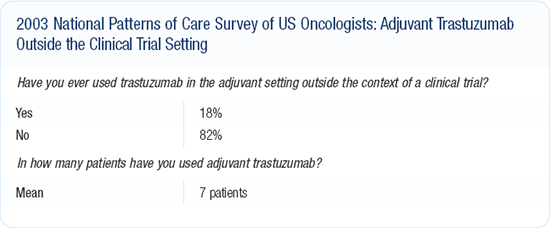
Although I would use adjuvant trastuzumab for such a patient who enrolled in a clinical trial, I personally would not use it in such a patient not enrolled in a clinical trial. No studies have shown that adjuvant trastuzumab is safe or tolerable, and it may just put the patient at risk. We all think adjuvant trastuzumab is going to work, but until we have clinical trial data showing that, I would not use it. We think we can do better, and maybe adjuvant trastuzumab will be one of the answers.
Select publications
|
