| You are here: Home: BCU 9|2003: George W Sledge, MD
Edited comments by Dr Sledge
Bevacizumab in the treatment of breast cancer
Although we do not have complete follow-up, the trial of bevacizumab in advanced colorectal cancer demonstrated approximately a five-month median improvement in overall survival. Will this translate to other diseases, including breast cancer? We don't know.
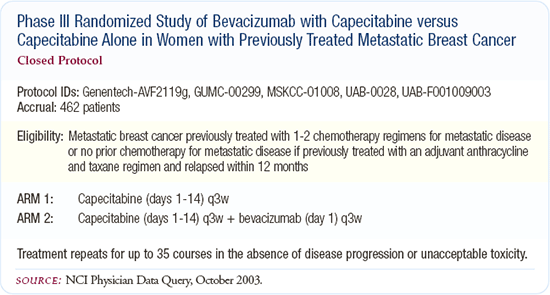
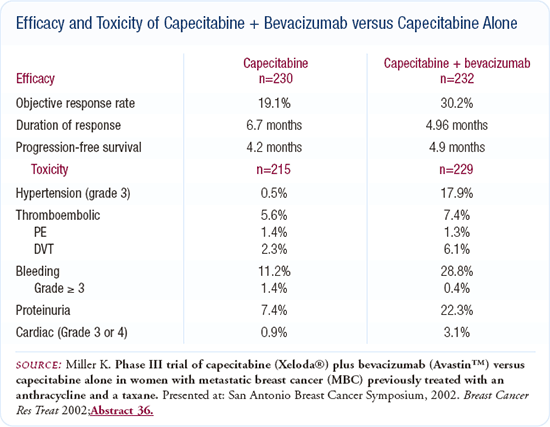
We do know that in breast cancer - in an anthracycline and taxane-refractory setting - adding bevacizumab to capecitabine nearly doubles the response rate but does not appear to improve time to progression or overall survival. So, while there is clearly a biologic impact in that setting, it is not clear that this is translated to real clinical benefit. It will be interesting to see whether using bevacizumab earlier in the metastatic breast cancer setting - as is being done in E-2100 - will provide a real clinical benefit, as opposed to just the response rate benefit seen in the trial of capecitabine and bevacizumab.
Relationship between Vascular Endothelial Growth Factor (VEGF) and HER2
Data has emerged suggesting that patients with HER2-positive tumors are more likely to have tumors that are positive for VEGF. Based on preclinical data, HER2 is upstream of VEGF, so a reasonable therapeutic hypothesis might be that co-blockade of HER2 and VEGF might result in greater benefit than blocking HER2 alone. This is currently being investigated at UCLA in a Phase I/II trial of bevacizumab and trastruzumab. I suspect that within a couple of years, we'll have some sense of whether this is a safe combination and whether it might provide some extra benefit.
Potential for bevacizumab in the adjuvant breast cancer setting
I believe it is reasonable to consider examining bevacizumab in the adjuvant setting. Approximately 30 to 50 percent of patients with breast cancer appear to have primary tumors that overexpress VEGF compared to surrounding normal tissue. In fairly large, albeit retrospective analyses, this population of patients had a higher rate of relapse, so there's a clear biologic hypothesis and rationale for exploring this strategy.
A major issue is whether or not we have the safety data yet to bring bevacizumab into the adjuvant setting. Should we wait for the results of E-2100? Should we start evaluating pilot approaches in the adjuvant setting? Should we start planning adjuvant trials? We are certainly considering these issues in the Eastern Cooperative Oncology Group.
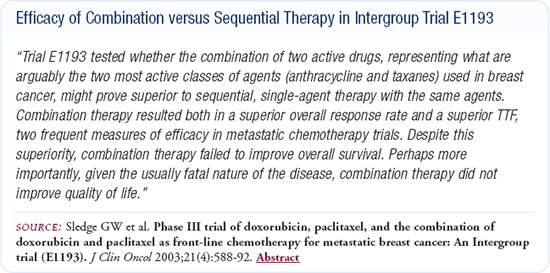
Combination versus sequential chemotherapy
ECOG-1193 compared doxorubicin followed by paclitaxel, paclitaxel followed by doxorubicin, and the combination of the two agents at initial relapse. The overall response rate for the combination of agents was better than that of either single agent. The time to treatment failure was approximately two months longer for the combination than for either single agent, but overall survival and quality of life were identical between the three arms.
My personal bias is this data provided support for the use of sequential single-agent chemotherapy. In my clinic, I find single agents to be less toxic in many cases, and I frequently offer the average patient with metastatic disease single-agent chemotherapy.

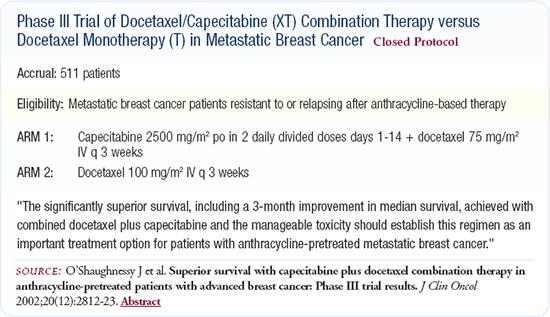
Joyce O'Shaughnessy's trial demonstrated a survival advantage of approximately three months for the addition of capecitabine to docetaxel in the metastatic setting for anthracycline-refractory patients. This was a well-conducted, statistically rigorous trial, and the results are certainly believable.
Capecitabine provides a real benefit for patients with metastatic breast cancer, but I don't conclude that combination therapy is superior to sequential single-agent therapy, and this trial did not test that hypothesis. There was no crossover arm from docetaxel to capecitabine or from capecitabine to docetaxel. In most cases, patients did not cross over to capecitabine. This trial is not comparable to ECOG-1193, which specifically looked at that question.

Fluoropyrimidines in adjuvant chemotherapy regimens
In the early 1990s, based on absolutely no data whatsoever, we dropped fluorouracil from adjuvant chemotherapy regimens in prospective randomized trials throughout the United States. There was a massive switch in clinical practice from FAC-type regimens to AC-type regimens.
Looking at the capecitabine data in the metastatic setting, one has to wonder whether or not that was an appropriate decision. Might an agent that improved survival in the metastatic setting also improve the cure rate in the adjuvant setting?
Management of patients with ER-negative, HER2-negative, metastatic disease
The majority of my patients have received adjuvant anthracycline-based chemotherapy. I am likely to offer single-agent sequential therapy - typically starting with a taxane - particularly if the patient relapsed fairly shortly after adjuvant therapy.
Based on the O'Shaughnessy data, I generally use capecitabine upon progression. If the patient wishes to continue chemotherapy after capecitabine, both gemcitabine and vinorelbine are capable of inducing remission in some patients in that setting and are reasonable options.
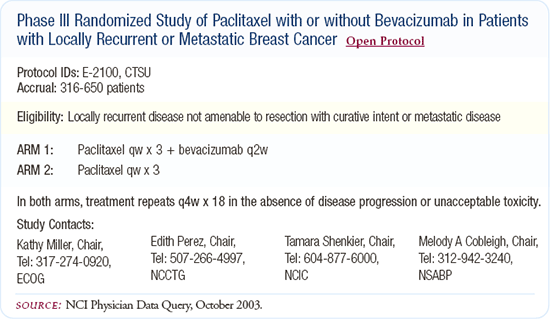
I also offer patients enrollment in clinical trials testing new concepts, including enrollment in E-2100, randomly assigning patients to weekly paclitaxel with or without bevacizumab. For patients who have received an anthracycline and a taxane in the adjuvant setting, we have little or no data. If the patient has relapsed within a year or so of adjuvant therapy, I am likely to offer single-agent capecitabine as first-line therapy.
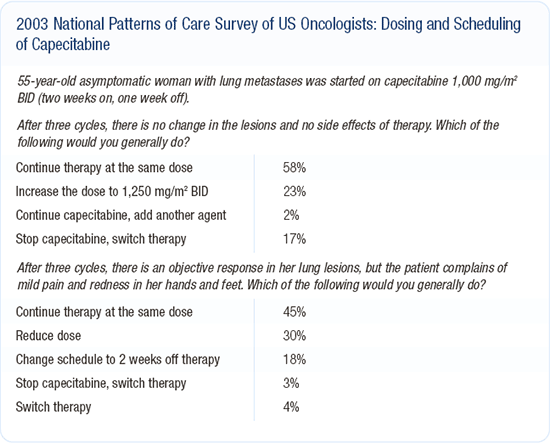
Dosing and scheduling of capecitabine
The FDA-approved dose of 2,500 mg/m2 in two divided doses, daily, is associated with a fair degree of hand-foot syndrome. I typically start patients at about 1,000 mg/m2 BID for 14 days on and seven days off.
We don't have much data to guide us here. Anecdotally, we've tried just about every dosing schedule imaginable to reduce toxicity. We've reduced administration from 14 out of 21 days down to 10 out of 21 days. We've lowered the dose and had patients receive treatment on weekdays with the weekends off. Depending on the patient, all three of these dosing schedules result in a lowering of toxicity.
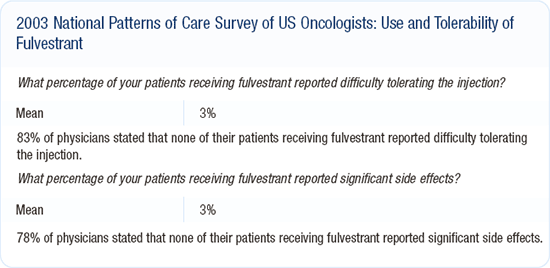
Efficacy and tolerability of fulvestrant
Like many of my colleagues, I'm not quite sure where to use fulvestrant, partly because we have limited clinical trial data. My interpretation of the results of the large North American and European trials is that fulvestrant and anastrozole are roughly equivalent agents in terms of survival.
In the North American trial, fulvestrant appeared to have some advantage over anastrozole in response and time to progression. My approach to therapy is to use survival to guide how I treat patients. The trials didn't demonstrate a survival difference, so I don't feel strongly that one agent is better than the other.
I use fulvestrant fairly regularly in my patients with steroid receptor-positive, metastatic breast cancer. I have patients who prefer receiving an injection once a month to taking pills every day. I have other patients who would prefer a pill to a shot. Aside from the acute discomfort of the injection itself, I've found fulvestrant to be an exceptionally well-tolerated medication.
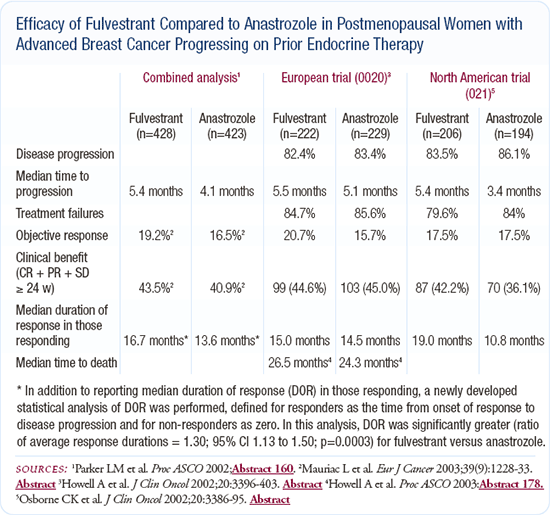
Counseling postmenopausal patients with ER-positive disease about adjuvant endocrine therapy
I routinely present the ATAC data when I counsel postmenopausal patients about adjuvant endocrine therapy. I say that both tamoxifen and anastrozole are FDA-approved drugs, and I consider both to be very reasonable options.
These discussions are more like negotiations than mandates, and patients frequently tell me what they prefer. To my surprise, I find that patients often make choices based on factors that would not have had a significant impact on my decision.
Women vary tremendously with regard to the toxicities they're willing to accept, and often their decisions are based on personal and family history.
Trials of combined blockade of growth factor receptors
We have a fair amount of preclinical data suggesting that combined blockade of growth factor receptors may be superior to blockade of one receptor. In the Eastern Cooperative Oncology Group, we're evaluating the combined blockade of both the epidermal growth factor receptor (EGFR) and HER2 using trastuzumab with gefitinib.
We're also evaluating combined blockade of both the EGFR and the estrogen receptor using gefitinib with either fulvestrant or anastrozole. We have good preclinical data for both of those approaches. Similarly, since we now know that patients with HER2-positive disease are more likely to overexpress VEGF, and studies in the metastatic setting are combining HER2 with VEGF blockade, using monoclonal antibodies for both.
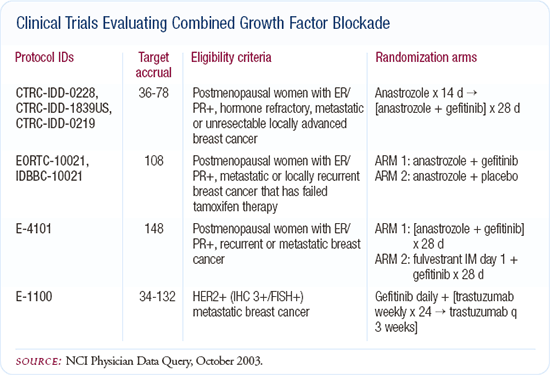
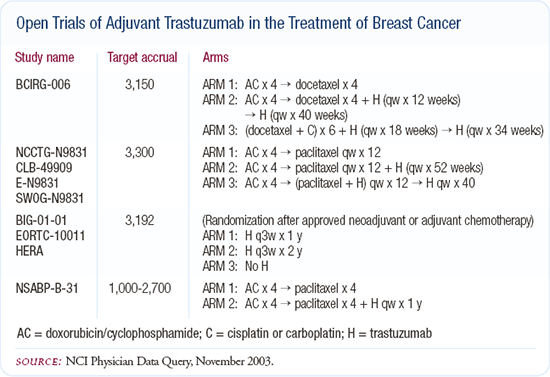
Clinical trials of adjuvant trastuzumab
In the adjuvant setting, the most interesting issue to me is HER2 blockade. We have four major ongoing randomized trials internationally, evaluating trastuzumab in combination with different chemotherapies or as a solitary blockade. If I were asked to place a bet, I would say that of the adjuvant trials we have available now, the HER2 trials are most likely to yield a positive result for overall survival.
Evolution of dose-dense chemotherapy
In the Intergroup, we are currently involved in a CALGB-led trial randomizing patients to one of the dose-dense arms of C-9741 versus another dose-dense regimen originally pioneered at the University of Washington by Bob Livingston and Julie Gralow. This dose-dense regimen, in essence, gives continuous chemotherapy during the course of the trial and looked very promising in an early, small dataset. So, we will be comparing two different forms of dose densification.
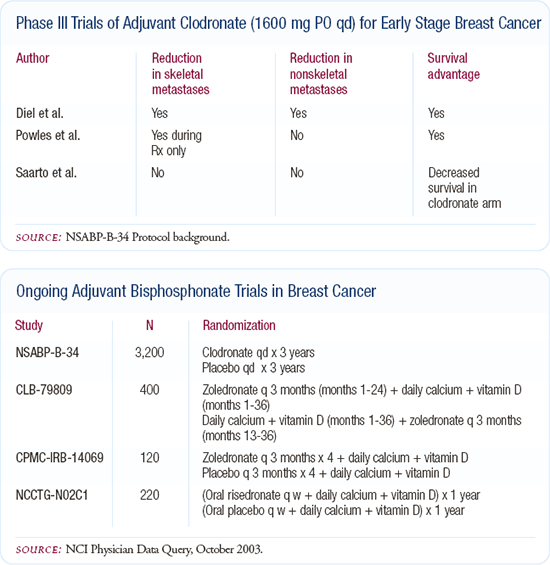
Adjuvant bisphosphonates
I'm fascinated by the trials of adjuvant bisphosphonate therapy. Two of the three European trials evaluating adjuvant clodronate suggested that it could lower the incidence of bony metastases. An interesting observation from the German trial was that bisphosphonates also diminished the likelihood of developing visceral metastases. This led to the hypothesis that, for some patients, bony metastases may represent a place from which other metastases may develop.
The NSABP has an ongoing, randomized trial assigning patients to receive adjuvant clodronate or not. In the very near future, an Intergroup trial will compare clodronate to other, more recent generation and more potent bisphosphonates.
From a toxicity prevention standpoint, adjuvant bisphosphonates may be very important. If, as the ATAC trial data suggests, use of an aromatase inhibitor results in perhaps a somewhat higher rate of fractures than tamoxifen, and if bisphosphonates prevent that problem, we might use them even if they don't improve survival. This is a very interesting strategy that needs to be pursued.
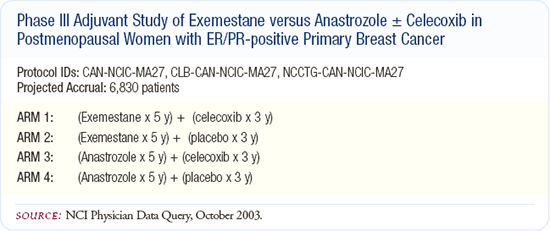
Intergroup trial of aromatase inhibitors and COX-2 inhibition
The first randomization of this trial is between the nonsteroidal aromatase inhibitor, anastrozole, and the steroidal aromatase inhibitor, exemestane. This trial asks whether or not there will be any efficacy, quality-of-life or toxicity differences between the aromatase inhibitors.
The second randomization of this trial is based on preclinical data suggesting COX-2 is upstream of aromatase in estrogen receptor-positive tumors. Tumors expressing a great deal of COX-2 have increased aromatase and are able to convert more androgens to estrogens. The same preclinical model system suggests that blocking both COX-2 and aromatase in mouse models results in greater benefits in treating existing cancers and preventing new cancers. This has led to a secondary randomization to either placebo or the COX-2 inhibitor, celecoxib.
Select publications
|
