| You are here: Home: BCU 9|2003: Sandra Swain, MD
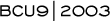
Edited comments by Dr Swain
NSABP-B-30: Adjuvant AC (doxorubicin/cyclophosphamide) followed by docetaxel (T) versus AT versus ATC
Trial rationale and design
When NSABP-B-30 was designed in 1997, taxanes were not routinely used in the adjuvant setting. Many of the investigators, including myself, believed that docetaxel was the most active agent in metastatic disease, and that it should be investigated in the adjuvant setting, which is why we included it in all three arms of B-30.
We also wanted to compare the various durations of treatment, so while the AC followed by docetaxel arm is a six-month treatment, the other arms are shorter in duration. The NSABP data showed four cycles of AC was effective, and we felt that four cycles of AT or TAC would be effective as well. Perhaps with hindsight, based on the TAC data, it would have been better to go with six cycles of TAC, but there's really no data showing six is superior to four cycles.
Initially we had several deaths in the ATC arm of B-30, probably due to the doses used - doxorubicin 60 mg/m 2, docetaxel 60 mg/m2 and cyclophosphamide 600 mg/m2. We changed the doses to those used in Nabholtz's regimen - doxorubicin 50 mg/m 2, docetaxel 75 mg/m2, and cyclophosphamide 500 mg/m2 - and since then we've had very few deaths.
We also changed the AT arm from doxorubicin 60 mg/m2 and docetaxel 60 mg/m2 to 50 mg/m2 and 75 mg/m2, respectively. The TAC regimen produced a high rate of febrile neutropenia - about 29 percent in the metastatic setting and 23 to 24 percent in the adjuvant trial - which we felt was unacceptable, so we added growth factors. It is up to the investigators whether they use the long- or shorter-acting growth factor.
Menopausal status and benefits of adjuvant chemotherapy
One of the most exciting factors we are evaluating in NSABP-B-30 is the impact of the patient's menopausal status. We have accrued approximately 4,400 patients, 88 percent of our target, and about one-half of the women were premenopausal when they began the trial. We are following their menses for at least two years after treatment and, while we pretty much know what to expect with arms containing AC, I know of no data on how the taxanes will impact menstrual function.
A critically important question is whether patients who experience amenorrhea have a survival benefit. The SOFT and TEXT trials are evaluating whether ovarian ablation, with either an aromatase inhibitor or tamoxifen, is beneficial, but right now we just don't know.
Hormonal therapy in NSABP-B-30
Initially, the only hormonal therapy patients received was tamoxifen, but when the ATAC data came out, we added an amendment to the trial allowing anastrozole in postmenopausal women if there's a contraindication to tamoxifen or the physician otherwise prefers to utilize the aromatase inhibitor.
We're still recommending - consistent with the ASCO Technology Assessment - patients receive tamoxifen. We were concerned allowing anastrozole would confound the results, but the statistician believed that our numbers were large enough that it would not likely affect the results.
Dose density data
The dose-dense data are early, but I doubt the benefit will disappear. The CALGB used a taxane that I feel is less effective than docetaxel, and many of us think the scheduling of the paclitaxel every two weeks rather than every three weeks is why the "dose-dense" therapy actually worked. We discussed whether, based on the dose-dense data, we should discontinue our B-30 trial, but I don't think it negates the questions we're asking. In our discussions regarding the next replacement trial, we are proposing a three-arm trial, possibly TAC versus dose-dense TAC versus dose-dense AC followed by paclitaxel/gemcitabine.
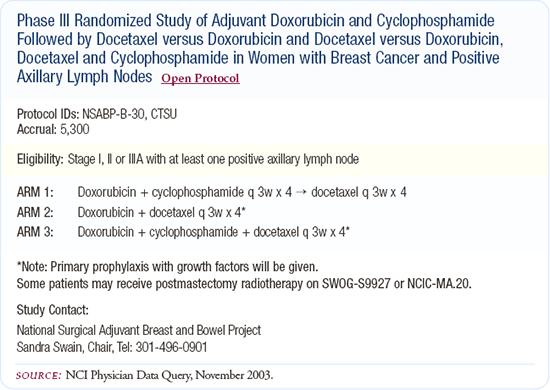
We need to determine predictive factors, look at gene profiles and find answers to our research questions more quickly, which is why the neoadjuvant trials are critical. We began designing B-30 six years ago and we won't have results for another four years. That's an extremely slow process and in the interim, things change as we get results from new studies. The NSABP B-27 replacement trial, a neoadjuvant trial looking at the microarrays before and after as well as switching the treatment schedules around, looks very interesting and I believe we'll be doing more of these important trials.

Adjuvant chemotherapy options
Off protocol, my first choice for treatment of younger patients with node-positive disease is TAC, which most of my patients choose and that probably reflects my bias. My second choice is the dose-dense regimen because the Phase III data shows a benefit, but I am concerned about the reported 13 percent incidence of blood transfusions. I've spoken with physicians who say it's not that high in actual practice, so it may not be a real effect, rather just a result of limited data.
My third choice is AC followed by docetaxel, because in NSABP B-27 we saw a higher pathologic complete response rate, although not a survival benefit. I don't use anthracycline-based regimens like FEC or CAF because I prefer a regimen that includes a taxane. Although data supports using these regimens in the pre-or postmenopausal patient, I'm convinced the taxanes provide an additive benefit.
Adjuvant trastuzumab
Trastuzumab is a fabulous drug that has made a huge difference for a lot of patients with metastatic disease and a very poor prognosis. We don't have any efficacy data for adjuvant trastuzumab, so I think it's unwise to use it in that setting outside of a clinical trial. I'm concerned about the potential cardiac toxicity, and we need the studies to mature in order to analyze the toxicity data. On the other hand, there are cases in which I would consider using trastuzumab, such as inflammatory breast cancer, where more of the patients are HER2-positive and survival is poor.
Neoadjuvant bevacizumab trial
We're studying neoadjuvant bevacizumab in inflammatory breast cancer, which has a lot of angiolymphatic invasion. Significant angiogenic growth factors may be present and stimulated by VEGF. We hypothesize that if we disrupt that stimulation, we'll have improvement in efficacy.
We have accrued 13 out of 20 patients. The patients receive bevacizumab for one cycle up-front, and undergo biopsies before and after treatment to look for gene changes. MRIs have shown decreased tumor vascular permeability in patients taking bevacizumab alone, and these patients say they can feel a change in their breast.
Time to progression, the primary endpoint in the bevacizumab/capecitabine metastatic breast cancer study, was negative; however, the response rate was 10 percent better in the bevacizumab arm. That is the same benefit seen in the colon cancer study. I think we'll find bevacizumab is active, which is why I'm continuing our neoadjuvant trial. I am still hopeful that we will see an efficacy benefit with this agent.
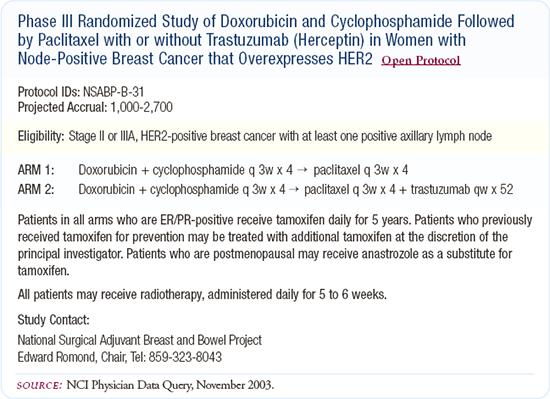
NSABP-B-31: Adjuvant AC followed by paclitaxel with or without trastuzumab
After the NSABP designed the adjuvant trial B-31, the Intergroup designed a similar trial so that the data could be analyzed together. I think that's great because it will be a stronger analysis. I hope we'll see a benefit with trastuzumab, which has been a miracle drug in the metastatic setting. If this trial is positive, there will still be a lot of scheduling questions to be answered such as, "How long do you really need trastuzumab and can it be administered every three weeks rather than weekly?"

Management of patients presenting de novo with HER2-positive metastatic disease
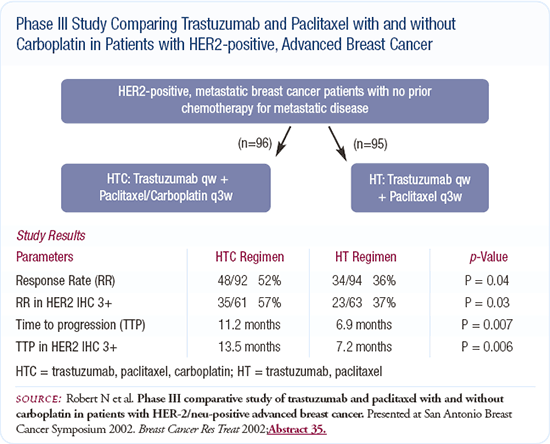
Since trastuzumab was approved for use, I've changed the way I treat patients presenting de novo with HER2-positive, metastatic disease. I no longer use an anthracycline first-line. Instead, based on Nick Robert's data, I use a docetaxel or paclitaxel/carboplatin and trastuzumab regimen. After the patient completes that therapy, I continue the trastuzumab and may later add vinorelbine. After that, I use capecitabine with trastuzumab. There was a Japanese group that showed either an additive or synergistic effect with trastuzumab and 5-FU, which supports using the two together.
I'm anxious for the data from the current doxorubicin HCl liposome injection/trastuzumab trials. If there's no cardiac toxicity, we may move that combination up front. In Slamon's trial, doxorubicin with trastuzumab had the best benefit, however, it also had the highest risk of cardiac toxicity, which is why no one uses it.
It doesn't surprise me that some physicians treat these patients with an anthracycline without trastuzumab. We were always taught that anthracyclines were the best drugs available, but based on my general experience and Dennis Slamon's data showing a survival benefit with the addition of trastuzumab and paclitaxel, I don't believe an anthracycline is the best choice.
Use of trastuzumab monotherapy
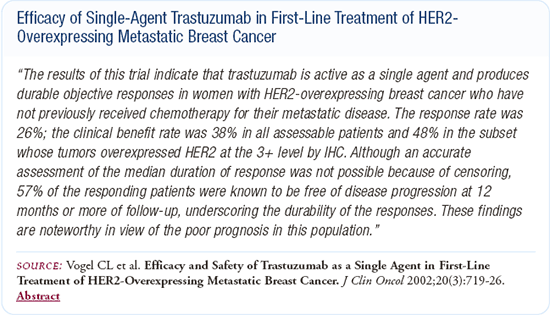
I've used single-agent trastuzumab in a couple of patients with limited metastatic disease, based on Chuck Vogel's data. One patient in her thirties with a cervical node recurrence experienced a complete remission in two or three months on trastuzumab. After almost a year of therapy, I took her off trastuzumab, hoping that if and when she had another recurrence, she would not be resistant to it. There's absolutely no data telling us how long one should remain on trastuzumab after a complete remission. You can make arguments either way, but we don't know the right answer.
Treatment of HER2-positive, ER-positive, metastatic breast cancer
I use hormonal therapy alone in these patients - generally an aromatase inhibitor if the patient is postmenopausal. I watch the patients carefully and if the disease progresses, then I move to trastuzumab. I've heard others say they would use trastuzumab up front, but we don't have any data showing a survival benefit in these patients. In addition, we know a lot of patients with hormone receptor-positive disease will do well for a long period of time, so I'm reluctant to add trastuzumab and make them come in every three weeks for IV therapy.
Advances in hormonal therapy
Aromatase inhibitors have dramatically changed hormonal therapy. I remember using aminoglutethimide, which had a lot of CNS toxicity, and megestrol acetate, which women hated because of weight gain. The aromatase inhibitors have very low toxicity, including exemestane, which may have a few more side effects but not much. I've used fulvestrant several times, but I used it later in the course of the disease so I've not seen as much efficacy with it. However, I think it's important to continue using this agent.
Treatment of HER2-negative metastatic breast cancer
I prefer sequencing single agents because I believe patients tolerate treatment better and live a better life. I want my patients to experience the most benefit with the least toxicity and, except for O'Shaughnessy's docetaxel/capecitabine trial, there's no evidence that combinations have a survival benefit. I've presented the docetaxel/capecitabine option to patients but they reject it saying they don't want all that toxicity for only a two-month median increase in survival. O'Shaughnessy also presented the gemcitabine/paclitaxel data showing a time-to-progression benefit with gemcitabine, but there's no survival data yet. In addition, neither of these two trials had a third arm where they sequenced the drugs.
In patients with HER2-negative, metastatic disease I often use capecitabine as my first-line therapy. It's a wonderful drug because it's very effective, and it's so well-tolerated. Patients don't mind taking a pill, and they love not losing their hair. It's important to watch the patients carefully and dose them appropriately to avoid hand-foot syndrome.
My second choice of therapy is a taxane. Like capecitabine, I think the taxanes have made a great contribution to improved survival in patients with metastatic disease. Paclitaxel came on board in 1992 and docetaxel in 1994, and, at least in the case of docetaxel, they are either equivalent or better than the anthracyclines.
I would also consider weekly doxorubicin or AC for the treatment of metastatic disease. I don't have a set treatment pattern, rather I look at the patient. If they don't have gastrointestinal symptoms, I consider capecitabine. If they do, then I consider an intravenous agent - probably AC or docetaxel.
Impact of improved supportive care
I've been practicing for 20 years, and I believe patients are surviving longer because we have more therapeutic options and better supportive care. Chemotherapy used to be miserable for patients, but now we have a number of antiemetics and growth factors that help patients tremendously. I'm not aware of any evidence that bisphosphonates increase survival, but they provide relief from fractures and pain. Zoledronate is easily administered in 15 to 30 minutes, relieves pain and it's great for the patients. Also, hair loss is a major issue for patients, especially in the metastatic setting where you just want to give them good quality of life. I use capecitabine as first-line therapy when I can, because patients don't experience alopecia and there's enough data showing good responses with the drug.
Select publications
|
